In an international study with an innovative design – a so-called adaptive platform trial – investigators were able to test multiple drugs at the same time and in a short time frame for the treatment of severe COVID-19, starting shortly after the outbreak of the corona pandemic. In the REMAP-CAP study, it was shown that corticosteroids, tocilizumab and sarilumab improve clinical outcomes in critically ill COVID-19 patients at the intensive care unit (ICU); and that high dose thromboprophylaxis improves outcomes in non-critically ill patients, but not in critically ill patients; and that antiplatelet therapy (aspirin, clopidogrel) and simvastatin are promising. In addition, convalescent plasma, lopinavir-ritonavir, hydroxychloroquine (or their combination), angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and vitamin C are ineffective or may worsen clinical outcomes.
COVID-19 has changed the landscape of clinical research. It evoked an unprecedented outpouring of activity to understand and treat a novel threat, reflected in close to 10,000 studies registered on clinicaltrials.gov, and hundreds of thousand publications in PubMed. It demonstrated the need of repurposing available treatments and optimizing supportive care, and highlighted the power of international research collaboration by independent research networks.
In previous virus outbreaks, due to practical, regulatory and legal hurdles the search for an effective treatment often could only start after the ‘hot phase’ of the epidemic was over. Partly thanks to the REMAP-CAP study, such investigations are now possible at a much earlier stage. REMAP-CAP is about how smart thinking after the 2009 influenza H1N1 pandemic has made it possible to start testing existing medicines for COVID-19, beginning shortly after the start of the outbreak. With the 2020 SARS-CoV-2 pandemic, the fear of many came to life: a new infectious virus that affects people worldwide and for which no vaccine or treatment exists.
Quick results needed
‘Why so late?’ ‘Is it possible to speed up the process?’ These are obvious questions asked at the start of an infectious disease outbreak. If existing drugs could be evaluated rapidly, patients would benefit already during an epidemic and we would be better prepared for future outbreaks. “Yes, identification of suitable drugs can be done quicker” says intensivist Lennie Derde, MD PhD at University Medical Center (UMC) Utrecht in the Netherlands. Together with physician-microbiologist prof. Marc Bonten, MD PhD, she coordinates the REMAP-CAP study in Europe. “This study was originally designed as an ongoing international study for patients hospitalized with community-acquired pneumonia (CAP) in intensive care units (ICUs). CAP is pneumonia contracted outside the hospital. Annually, around 3 million people worldwide die from CAP and an estimated one quarter of all hospitalized pneumonia patients end up in the ICU.”
If a particular drug shows better results, a larger percentage of new patients will be allocated to that drug. Hence fewer people are exposed to less promising medicines.
Adaptive platform trial
One goal of the REMAP-CAP study is to test different types of interventions for effectiveness in CAP. The various treatment options are selected on the basis of an estimate of how serious the disease is and what the possible pathogens are. REMAP-CAP is a so-called Adaptive Platform Trial (REMAP stands for Randomized, Embedded, Multifactorial, Adaptive Platform), a clinical study with an innovative design that allows to test multiple medicines concurrently (for study design details, click here). Lennie: “This is a randomized trial with a control group, but smarter and more flexible. In a traditional clinical study, patients receive one treatment from a short list of alternatives – usually one or two. In REMAP-CAP we can test several groups of medicines in parallel. We also have faster results: in a regular study, one works for years towards the end point at which the results become clear and one cannot look ‘under the hood’ in the meantime. An adaptive platform trial is specifically designed to perform frequent adaptive analyses, making sure one gets to a conclusion about the effectiveness of an intervention as soon as possible, and allows more people to be randomized to the more promising options during the trial. If a particular drug shows better results, a larger percentage of new patients will be allocated to that drug. Hence fewer people are exposed to less promising medicines. As a result, patients are more likely to benefit from the knowledge that is gradually yielded by this type of study.”
If there is an outbreak and the patients come in, participating hospitals must be ready to start the trial immediately.
Five years of preparation
The idea for REMAP-CAP was conceived after the 2009 H1N1 influenza pandemic (also called ‘Mexican flu’ or ‘swine flu’) and preparations for the trial started in 2014 (with funding from the European Commission via the PREPARE project). The study had a preparation time of no less than four years until 2018 when the study started and eventually ran in ICUs in Europe (including the Netherlands), Australia, New Zealand and Canada. The first patients with CAP were enrolled in 2018 at UMC Utrecht. It took four years of preparation during which all kinds of obstacles in design, regulatory and legal aspects as well as contracting had to be resolved to enable this unique international cooperation. Lennie: “If there is an outbreak and the patients come in, participating hospitals must be ready to start the trial immediately. They should then be able to start assigning patients to different treatment options right away to get valid results as soon as possible.”
Outcomes from REMAP-CAP
In addition to long-term pneumonia research, REMAP-CAP was also designed to be immediately adaptable in the event of a pandemic. “Most pandemics stem from viruses that cause patients to develop pneumonia,” says Lennie. “Thanks to the design and international collaboration of ICUs, REMAP-CAP offers the large-scale infrastructure to be able to conduct scientific research into potentially effective drugs immediately after the start of the outbreak. As a result, the first COVID-19 patient could be included on March 9, 2020, less than 6 weeks after the WHO declared COVID-19 a Public Health Emergency of International Concern, and 2 days before declaring it a pandemic.” So far, REMAP-CAP has randomized worldwide more than 14.000 unique patients (of which more than 10.000 were diagnosed with suspected or proven COVID-19), investigating 66 (ongoing or completed) interventions at close to 300 sites, and, in addition to the regions mentioned above, is now also active in the United States, India, Nepal, Pakistan, Colombia and Japan.
Since the start of the pandemic, REMAP-CAP has provided several urgently needed answers with immediate impact on the treatment of hospitalized COVID-19 patients.
COVID-19 patients often also suffer from pneumonia and the most serious cases are admitted to the ICU. Lennie: “For COVID-19, the possibility of testing multiple medicines in parallel as with REMAP-CAP means that we investigated for example which antivirals, which anticoagulants and which immune modulators worked best. Since the start of the pandemic, REMAP-CAP has provided several urgently needed answers with immediate impact on the treatment of hospitalized COVID-19 patients. The first results from the REMAP-CAP study could already be published in September 2000, approximately six months after the outbreak was declared a pandemic. An overview of the main outcomes so far is presented below.
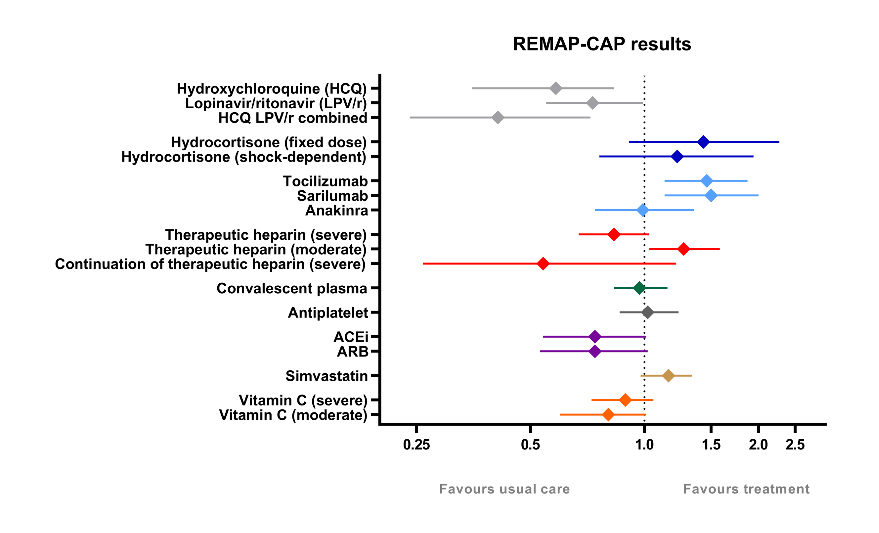
REMAP-CAP Results overview
Hospitalized critically ill COVID-19 patients:
- Corticosteroids (JAMA, September 2020), tocilizumab and sarilumab (NEJM, February 2021) improved outcomes;
- Antiplatelet therapy (JAMA, March 2022), and simvastatin (NEJM, October 2023) may improve outcomes;
- High-dose thromboprophylaxis, interferon-β and anakinra (NEJM, August 2021) and convalescent plasma (JAMA, October 2021) were ineffective;
- Lopinavir-ritonavir (Intensive Care Medicine, July 2021), angiotensin-converting enzyme inhibitor and angiotensin receptor blocker (JAMA, April 2023) and vitamin C (JAMA, October 2023) likely worsened outcomes;
- Hydroxychloroquine (Intensive Care Medicine, July 2021) and continued high-dose thromboprophylaxis (Intensive care Medicine, May 2023) worsened outcomes.
Hospitalized non-critically ill COVID-19 patients:
- High-dose thromboprophylaxis improved outcomes (NEJM, August 2021);
- Vitamin C was ineffective (JAMA, October 2023)
Adaptive platform trials (APTs) such as the REMAP-CAP study will continue to generate increasing interest because they support two rising developments in healthcare: (1) patient-centered precision medicine and (2) the learning health system. APTs are purpose-built to efficiently test multiple interventions in multiple disease subtypes, a key thrust of precision medicine. The APTs’ perpetual nature, with use of features such as responsive-adaptive randomization, also provides a strategy for continuous quality improvement with respect to the comparative effectiveness and adoption of existing interventions, a key thrust of the learning health system. For detailed information about the REMAP-CAP study, go to www.remapcap.eu or www.remapcap.org.
Previous articles on this subject
October 25, 2023: REMAP-CAP study presents results to guide care of severely ill patients with COVID-19 using simvastatin and Vitamin C.
March 22, 2022: Aspirin may improve 3-month survival for patients critically ill with COVID-19.
August 5, 2021: High doses of blood thinners are effective for hospitalized COVID-19 patients, but not for ICU patients.