Imagine a future where intensive care and long-term hospitalizations are a thing of the past. Thanks to image-guided treatments, this is no longer a distant dream. This story will take you on a journey through the world of advanced medical imaging technology, where every treatment counts for a healthier future.
There is a threat of major shortages in healthcare, especially in terms of staff. In a few years, there may not be enough caregivers to provide treatments and staff the intensive care units. Due to the growth and aging of the population, the demand for care will only increase. How can we overcome this problem?
“I sincerely believe that we can make a difference with our research and efforts,” says Nico van den Berg, professor of computational imaging at UMC Utrecht. “Image-guided treatments are not the complete solution to the expected healthcare infarction. They do play an important role in keeping healthcare accessible to as many people as possible.”
Nico van den Berg is professor of computational imaging at UMC Utrecht. In his research, he improves MRI techniques so that patients receive more accurate diagnoses and more effective treatments. Lenny Verkooijen is professor of evaluation of image-guided treatments at UMC Utrecht. Lenny focuses on efficient and patient-friendly methods to test new image-guided treatments.
What are image-guided treatments?
Image-guided treatments view and treat a disease at the same time. They use detailed images along with treatment methods. The images come from an MRI or CT scan, for example.
The images are leading in the treatment. They accurately visualize an abnormality, such as a tumor. Subsequently, the abnormality is given a very targeted treatment.
Image-guided treatments are generally less invasive than traditional surgery. They put much less strain on the body. We call this a ‘non-invasive treatment’. No cuts or surgeries are required.
A long hospital stay is therefore often unnecessary. Also, there are usually fewer side effects. That makes it a lot more pleasant for the patient.
What are the benefits?
“Image-guided treatments offer many advantages,” says Lenny Verkooijen, professor of evaluation of image-guided treatments at UMC Utrecht. “When you are going to irradiate a tumor, you want to destroy the cancer cells with a high dose of radiation. At the same time, you want to do as little damage to the surrounding tissue as possible. The image-guided treatment provides a very precise picture of the tumor. As a result, the irradiation is very accurate. In this way, the tumor receives an effective treatment. There will also be fewer side-effects and the patient will recover faster. And fewer treatments are needed. Of course, those are big pluses.”
“Take, for example, the treatment of prostate cancer,” Nico adds. “Because we have improved the images, the treatment is now much shorter. Men with prostate cancer first had visit the hospital 20 times. That’s only 5 times now. Some people even schedule their radiation treatment at the end of their working day. Of course, being sick and exposed to radiation has an impact on your life. It’s not something you ‘just’ do. But how pleasant is it that you don’t have to stay in the hospital? With these treatments, many patients can go on with their daily lives. That’s a huge win.”
“With our new imaging techniques, we can now also irradiate tumors that could not be treated with radiotherapy before.”
More tumor types can be treated
“With our new imaging techniques, we can now also irradiate tumors that could not be treated with radiotherapy before,” says Lenny. “A good example is pancreatic cancer. This type of cancer is difficult to treat and often has a poor prognosis. If you were to irradiate a pancreatic tumor in the old way, you would soon damage the healthy tissue around it. A lower dose of radiation doesn’t work well either.
With our latest imaging techniques, we get a razor-sharp picture of the tumor and the surrounding tissue. We can give a strong dose of radiation in a safe way. Thanks to image-guided treatments, people with pancreatic cancer are now more likely to live longer.”
Affordable care
Our total healthcare system can also benefit from image-guided treatments. For instance, financially. “Take, for example, the difference between the traditional radiation treatment for prostate cancer with the CT scan and the MR-Linac,” Lenny explains. With the CT, it often takes 5 to 20 sessions to irradiate prostate cancer. With the MR-Linac, it can be done in 2 to 5 irradiation sessions. Irradiation with the MR-Linac is more expensive, but the total costs are lower due to fewer sessions.”
Medical technology often has an expensive image. This is not always justified. Half of all people with cancer receive radiation treatment. The costs are about 5 to 10% of the total cancer care budget. This makes radiation an affordable option, especially compared to some expensive cancer drugs. Lenny: “We want to develop effective and cost-efficient treatments. By doing so, healthcare will remain accessible to everyone.”
Regionally united in Oncomid
To also make cancer care more effective and efficient, UMC Utrecht and surrounding hospitals work together in Oncomid. “Cancer care is complicated,” Lenny says. “Many patients receive most of their treatment in the hospital near them. Oncomid is building a bridge between UMC Utrecht and these local hospitals. Patients come to us for special, complicated treatments, such as radioembolization or radiation with the MR-Linac. Then they go back to their own hospital.”
Collaboration between the hospitals is key. “Together, we provide the best care,” Lenny explains. “We also conduct joint research. A good example is our collaboration with the urologists of the Antonius Hospital. Together, we are, for instance, investigating when it’s best to choose surgery and when to opt for radiation as the best treatment.”
“We are pioneers, both in the Netherlands and worldwide. We are always looking for new techniques and often work together with external parties to make that possible.”
UMC Utrecht as a pioneer
UMC Utrecht holds a special position in image-guided treatments. “We are in a special position,” says Nico. “Actually, we are pioneers, both in the Netherlands and worldwide. We are always looking for new techniques and often work together with external parties to make that possible.”
“Not only Utrecht, but the whole of the Netherlands has put itself on the map with image-guided treatments”, Nico continues. “Our techniques, such as radiology, MRI and ultrasound, are widely recognized internationally. We make a major contribution to international conferences. It seems as if the Netherlands is much bigger than it actually is.”
“The development of the MR-Linac is a real milestone,” Nico states. “It is so unique that we have developed such an advanced device, here in Utrecht. I still remember the days in the lab, when we were putting components together. Now, more than 140 organizations worldwide possess an MR-Linac.”
UMC Utrecht’s image-guided treatments
An overview of the most important image-guided treatments that researchers and physicians at UMC Utrecht are working on:
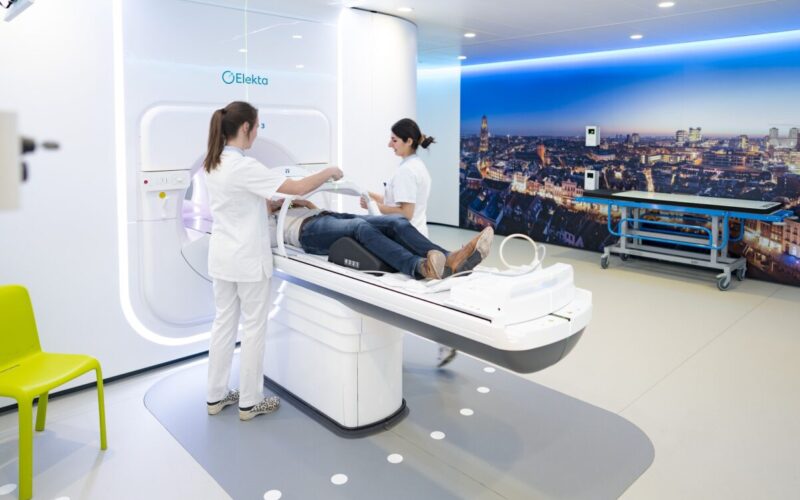
MR-Linac
The MR-Linac is a combination of an MRI scanner and a radiotherapy machine. By combining these two devices, the MR-Linac can take images of the body as well as irradiate cancer cells. This allows doctors to see exactly where the tumor is located while irradiating the tumor. They can also target the radiation to the tumor more precisely. This means that the cancer cells receive an effective treatment, while the healthy tissue suffers less damage. This is particularly useful for tumors that move (e.g. due to breathing or intestinal peristalsis) or for tumors that are close to important organs.
The MR-Linac was invented and developed at UMC Utrecht. Since 2018, the device has been used in the clinic, especially in the treatment of cancer. It can help with various types of cancer, such as prostate, breast, and lung cancer. MR linacs ar now being used worldwide. This image-guided treatment has already helped many patients.
MR-HIFU
MR-HIFU stands for Magnetic Resonance – High-Intensity Focused Ultrasound. That’s quite a mouthful, so let’s break it down step by step:
- Magnetic Resonance (MR): as with the MR-Linac, this part refers to the MRI scanner. The device first creates a detailed image of the area to be treated.
- High-Intensity Focused Ultrasound (HIFU): this technique focuses concentrated ultrasound waves (sound waves) on a specific point in the body. When these waves converge at that particular point, they produce heat to destroy the tissue.
Doctors use the MR-HIFU for various diseases. For example, in the case of prostate cancer, to treat a tumor that is still small. The technique also works well to remove fibroids (fibroids). These are benign tumors in the uterus. And if cancer has spread to the bones, the MR-HIFU can help reduce the pain by destroying the cancer cells. In addition, research is underway into the use of MR-HIFU for other diseases, including other types of cancer.
Radioembolization
Doctors mainly use this technique for tumors in the liver. They use radioactive spheres that can irradiate the tumor from the inside. These spheres are tiny particles that contain a radioactive substance. They are so small that they can travel through blood vessels to the cancer cells.
Radioembolization is particularly useful for the treatment of liver cancer and metastases in the liver. It is a targeted way to destroy cancer cells with minimal damage to healthy tissue.
Nuclear therapies
These are treatments that use radioactive substances to treat diseases. The radioactive substances emit radiation. That radiation destroys the cancer cells.
One difference with radioembolization is that nuclear therapies work throughout the body, and not specifically in one place. The radioactive substance is linked to a protein. That protein goes in search of cancer cells very specifically. Once it reaches the cancer cells, the radioactive substance emits the radiation.
Doctors use nuclear therapies especially for cancer. For example, in thyroid cancer, neuroendocrine tumors and metastatic prostate cancer. This treatment can also help with pain in the bones, if the cancer cells have spread to the bones.
The power of AI
Nowadays, artificial intelligence (AI) is becoming more and more significant in healthcare. “With AI, we can make diagnoses faster and more accurately,” Nico says. “During image-guided treatments, AI acts as an assistant, reading and interpreting images.”
Nico’s enthusiasm grows when he talks about image-guided radiation. “Here, AI is a real game changer. It adjusts the radiation in real-time, based on the images we take of the patient at that moment. This allows us to continuously adjust the treatment to what the patient actually needs. But it doesn’t stop there. After the treatment, AI will help us to determine how intensively we are going to monitor a patient. AI can even advise us on which follow-up treatment will be most suitable.”
“The impact of technology on how we work is really fascinating,” Lenny adds. “Previously, a radiation oncologist made a radiation plan. Now, algorithms are making these plans, thanks to very progressive imaging.”
Lenny paints a picture of the future: “Just imagine: a machine scans a patient, recognizes the tumor and makes a radiation plan. That’s the innovative direction we’re heading at. This innovation will reduce the pressure on medical staff. Given the current shortage of healthcare personnel, I think that would be very welcome.”
Vision for 2030
What does the future have in store for us, regarding image-guided treatments? “We want to offer more and more of these treatments,” Nico says. “By 2030, I hope we will have replaced part of intensive care with minimally invasive image-guided treatments. Perhaps, by then, they will no longer be invasive at all. That would be a great way to alleviate the pressure on our healthcare system.”
“Imaging helps us to quickly choose the right treatment for a patient,” Lenny adds. In this way, we will personalize healthcare even more. Automation and AI will play a major role in this. By 2030, technology will have taken over many tasks from lab technicians and radiation oncologists.”
Nico emphasizes that support will hve to come from multiple sides. “It is very important that health insurers and the Ministry of Health recognize this development. It’s not just about the patient, it’s about the entire healthcare system. In doing so, we want everyone, from healthcare providers to patients, to understand what image-guided treatments entail. Good communication is key here.”
His eyes sparkle when he talks about the plan to make this all happen. “I envision us as an innovation hub. A place where we are constantly developing new image-driven methods and techniques. And not just us researchers. That is, in this hub, we will work together with doctors, universities and companies.”
Making innovation more sustainable
Such an innovation hub is important for rapid advances in image-guided treatments. “In the past, universities and university hospitals mainly worked with large companies, such as Philips and Elekta,” Lenny explains. “This cooperation is very useful, but it is not enough. If we only work with such companies, it slows down the speed at which we can innovate.”
She emphasizes that different areas of expertise and backgrounds are needed. “Innovation requires more than smart engineers, scientists and doctors. We also need regulatory knowledge and an understanding of artificial intelligence.”
Lenny also has concerns. “After many projects, valuable knowledge is lost. Many colleagues keep reinventing the wheel. That breaks our efficiency. Researchers need a lot: investors, data, new technology. At each stage, a new partner is added. That takes time, and we lose knowledge in the meantime. That has to change.”
How to develop an image-guided treatment?
The development of an image-guided treatment is a careful process, which involves many steps. Below, you can see what is involved to create such an innovation:
- From idea to concept. Scientists start with an idea. This may stem from their research, or from an emerging need in the healthcare industry. They refine this idea into a proof of concept. This will be tested in a laboratory setting to see whether the idea will really work.
- Share and protect. If the concept works, the researchers will publish their findings, sharing their knowledge with colleagues all over the world. At the same time, they will protect their idea through intellectual property (IP), preventing others from running off with it.
- Demonstration and validation. The researchers will then demonstrate the concept in a controlled environment. After this demonstration, they further validate the technology in the laboratory.
- Out of the lab. After successful laboratory tests, the investigators will test the technology in a real clinical setting. This means that patients will now be directly exposed to the new technology. This will allow the researchers to measure efficacy and safety.
- Collaboration & Expansion. In case of positive clinical results, the investigators will extend the trajectory. They will involve professionals from a wide range of disciplines to improve the technology, ensuring that everything will run smoothly.
- Business community joins in. When all the previous steps went well, a commercial company will adopt the technology. They will ensure that the technology will be integrated into software and distribute it to clinics all over the world.
- Continuous improvement. Even when the image-guided treatment is available to patients, the research will continue. Scientists will keep on evaluating how the treatment is going and what should be improved.
Despite these clear steps, the development process is not easy. As with drug development, many newly developed medical technologies do not make it to the end.
“We want to take the further development of image-guided treatments to the next level. That is why we have set up the ‘Imagine Open Innovation Lab’. In this environment, companies, academics, hospitals and start-ups can work together very efficiently.”
A new way of collaborating
Lenny says that UMC Utrecht has a clear vision. “We want to take the further development of image-guided treatments to the next level. That is why we have set up the ‘Imagine Open Innovation Lab’. In this environment, companies, academics, hospitals and start-ups can work together very efficiently.
“For real breakthroughs, we need capital, expertise and entrepreneurship,” Nico adds. “All those involved in developing image-guided interventions must come together. The goal is simple: to share knowledge and promote collaboration. That’s how we will make innovation go smoother and smoother. For instance, when we make images for MRI-guided therapy, we can also use them for MRI-guided HIFU therapy. This allows us to work faster and smarter.”
Lenny emphasizes the importance of building a strong foundation. “This way, all parties involved can learn and grow. Sharing knowledge and resources strengthens the entire sector. This allows us to innovate faster and more powerfully. By directly involving medical professionals, we ensure that our solutions actually respond to the needs in practice. If a clinic in Europe needs a special device, we’re there. Our experience, especially with AI, makes all the difference.”
“Innovation alone is not enough. There must be a focus on implementation. Through training, workshops and other methodologies, we can accelerate adoption. At the end of the day, it’s all about impact.”
A new way of collaborating
Lenny says that UMC Utrecht has a clear vision. “We want to take the further development of image-guided treatments to the next level. That is why we have set up the ‘Imagine Open Innovation Lab’. In this environment, companies, academics, hospitals and start-ups can work together very efficiently.
“For real breakthroughs, we need capital, expertise and entrepreneurship,” Nico adds. “All those involved in developing image-guided interventions must come together. The goal is simple: to share knowledge and promote collaboration. That’s how we will make innovation go smoother and smoother. For instance, when we make images for MRI-guided therapy, we can also use them for MRI-guided HIFU therapy. This allows us to work faster and smarter.”
Lenny emphasizes the importance of building a strong foundation. “This way, all parties involved can learn and grow. Sharing knowledge and resources strengthens the entire sector. This allows us to innovate faster and more powerfully. By directly involving medical professionals, we ensure that our solutions actually respond to the needs in practice. If a clinic in Europe needs a special device, we’re there. Our experience, especially with AI, makes all the difference.”
“But innovation alone is not enough,” says Nico. “After development and clinical evaluation, there must also be a focus on implementation. You can’t just introduce a new technology without guidance.” He sees a key role for education. “Through training, workshops and other methodologies, we can accelerate adoption. At the end of the day, it’s all about impact.”
Introducing the cockpit
“It’s also important to increasingly start sharing expertise remotely,” says Lenny. “This will allow us to speed up the implementation of new techniques, without having to be physically present in other centers. We will be able to achieve this by means of a ‘cockpit’: a room with advanced workstations and screens.”
“In this innovative setting, our clinicians will remotely connect to another clinic, somewhere else in the world,” she adds. “We will watch live videos and share real-time images. And we will observe treatments and provide immediate feedback in a very precise way, even pinpointing certain areas on scans.”
“We would like to train medical professionals around the world in new image-guided techniques”, Lenny emphasizes. “With the help of the cockpit, they will broaden their expertise and continue to provide excellent care to their patients.”
The future in sight
“Everything we do here with image-guided treatments should ultimately benefit the patients. Of course, that is and will remain our focus,” says Nico. “Even if they get a serious diagnosis, I hope they will be able to get as much out of life as possible. It’s all about quality of life. The moments you share with your loved ones, that’s what makes life valuable.”
“Our goal for the next 10 years is clear”, Lenny adds. “We would like to achieve fewer hospital admissions, more minimally invasive treatments and more efficient workplaces for healthcare professionals. That’s quite a challenge, but crucial for the future of healthcare.”