A groundbreaking approach that targets the degradation of membrane proteins in cancer cells has received funding from the European Innovation Council (EIC) Pathfinder program. The goal? To target previously “undruggable” cancer-related proteins by selectively degrading them and potentially offering a new way to overcome resistance to current cancer therapies.
Led by Madelon Maurice at the Center for Molecular Medicine, UMC Utrecht and the Oncode Institute, the OutMARCH project brings together experts in AI-based protein design, cancer biology, and antibody development. The team will work on the development of so-called SureTACs – bispecific antibodies designed to specifically degrade surface proteins that drive cancer growth. This technology could offer a more effective and targeted treatment option compared to traditional therapies, which are often limited due to resistance and side effects.
The OutMARCH team aims to unlock the potential of MARCH E3 ligases, specialized enzymes that can target and degrade specific proteins on cancer cells. By bringing these enzymes in close proximity to a target protein, the team aims to develop a therapy that can selectively attack cancer cells while minimizing damage to healthy tissue. A major technical challenge is to develop drugs that bind this class of enzymes. If successful, this approach may address some of the key challenges in oncology, including resistance to existing cancer treatments and the on-target off-tissue effects that can cause harm to healthy cells.
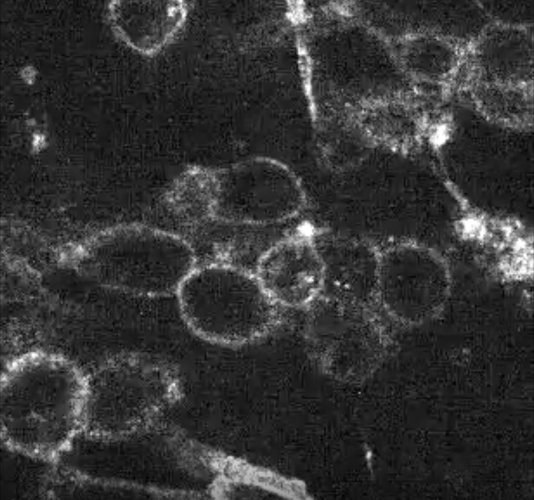
Untreated cells - in white the 'target' protein on te cell surface.
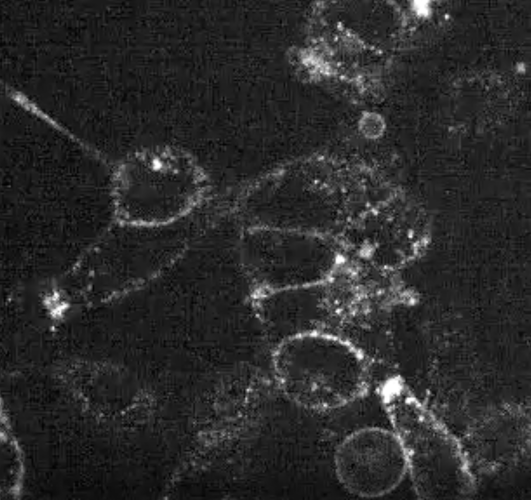
Cells treated with SureTACs - the protein completely disappears after treatment starts.
While the project is still in its early stages, it has the potential to transform cancer treatment. By targeting previously inaccessible cancer proteins, the technology may open the door to more personalized and effective therapies in the future.
“Our team is thrilled to work together and unlock the full potential of SureTACs technology. Our high risk-high gain project holds promise for novel cancer treatment strategies by focusing on both precision targeting and overcoming treatment resistance. This approach could help shape the next generation of personalized cancer therapies.”
The team’s long-term goal is to develop a scalable platform for protein degradation that can be used to treat a wide range of diseases, including cancer, autoimmune conditions, and chronic inflammation. This research holds potential to move beyond the laboratory, offering the possibility of clinical applications in the future.
The OutMARCH project brings together a diverse and interdisciplinary team of researchers. The project is led by prof. Madelon Maurice from UMC Utrecht and Oncode Institute and involves key collaborators Dr. Danny Sahtoe (Hubrecht Institute and Oncode Institute), Prof. Florian I. Schmidt (University Hospital Bonn and University of Bonn), Prof. Thorsten Zenz (University of Zurich), and the biotech startup Laigo Bio.
Together, these experts in cancer biology, protein design, antibody development, and clinical research are working to advance the development of SureTACs technology. Through their interdisciplinary collaboration, this innovative approach will be employed to tackle challenging cancer types, including therapy-resistant B-cell lymphomas and gastrointestinal cancers.
The key objectives of the OutMARCH project include: