In recent times, there has been growing interest in the potential of stem cell therapy. Stem cells possess the remarkable ability to self-renew and differentiate into specialized cells, offering hope for addressing various medical conditions. At UMC Utrecht, we aim to use stem cells in the understanding and treatment of (rare) diseases, but we also invest in the unique logistical and production aspects of stem cell therapy by developing new facilities and overcoming regulatory challenges.
Stem cells to repair brain damage in newborns
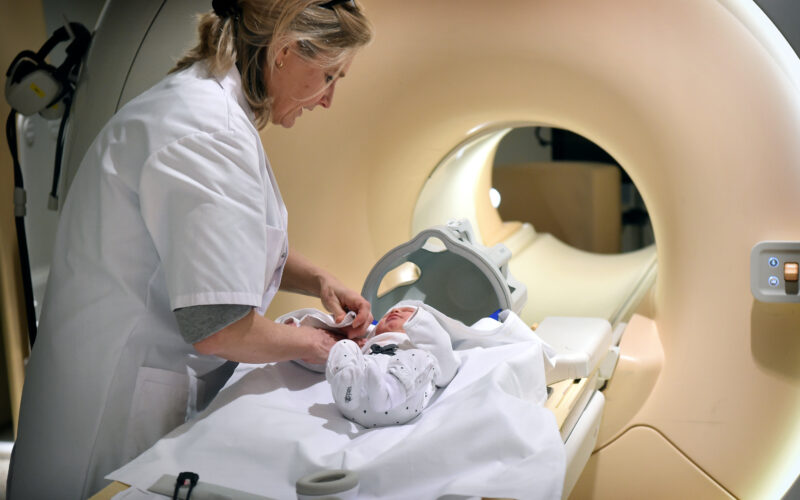
Utrecht researchers and doctors are developing a new form of stem cell therapy for newborns with brain damage after a cerebral infarction. The treatment aims to support the natural recovery ability of the baby’s brain. The clinical study, recently completed, indicates that mesenchymal stem cells can be safely administered to these newborns via nasal drops. The findings of the research groups led by Cora Nijboer and Manon Benders have been published in The Lancet Neurology.
The intended new treatment targets newborns with brain damage caused by an infarction, which can result from the blockage of a blood vessel. It is known that these children will experience problems later in life, depending on the specific area of the brain that is damaged. Symptoms they may develop include motor problems, learning difficulties, behavioral and concentration problems, and epilepsy.
“From our lab experiments, we deduced that treating within a few days after the blockage of a blood vessel is feasible by administering stem cells via the nose. We have demonstrated in animal and cell models that there was restoration of damaged brain tissue, a process called neuroregeneration.”
How stem cells evoke self-repair of the brain
Neuroscientist Cora Nijboer explains, ‘From our lab experiments, we deduced that treating within a few days after the blockage of a blood vessel is feasible by administering stem cells via the nose. We have demonstrated in animal and cell models that there was restoration of damaged brain tissue, a process called neuroregeneration.’ This treatment involves administering so-called ‘mesenchymal’ stem cells with the aim of supporting the affected parts of the brain, thus stimulating the baby’s own brain stem cells to repair the damaged tissue.
Nijboer explains the mechanism: ‘The damaged area in the brain sends out ‘distress’ signals. This attracts the mesenchymal stem cells to the damaged spot in the brain, where they produce substances that activate the healing mechanism of the brain’s own stem cells.’ Manon Benders, professor of neonatology, adds, ‘This restoration is particularly possible in newborns because the brain and its networks are still undergoing significant growth and development from the third trimester of pregnancy and throughout the first months of early life. Through stimulation, even in damaged tissue, remaining vital connections can be repaired through neuroplasticity.’
How are the cells administered?
The stem cells are administered through nasal drops. When the research began in 2010, few researchers believed that the cells could reach the damaged spot in the brain this way. However, after years of work by multiple researchers, Nijboer’s team now better understands the route the stem cells take in just a few hours. ‘They pass through a kind of sieve bone in the nose and are then taken up into the blood vessels of the brain membranes or the cerebrospinal fluid. This way, they migrate to the site of brain damage within a few hours,’ Nijboer explains.

First results in a small group
Nijboer’s team, along with Manon Benders’ clinical team, brought the treatment to patients for a safety trial. Ten newborns who suffered a cerebral infarction participated in the study. As soon as a cerebral infarction was detected on an MRI scan, they were given a one-time dose of stem cells through nasal drops. Around the age of 3 months, the researchers performed another MRI scan to assess the extent of remaining damage after the infarction. The development of the babies was then monitored at the neonatology outpatient clinic. Benders: ‘In the data from this safety study, we saw signs of the treatment’s effectiveness. However, we need to thoroughly investigate its efficacy in a larger group of newborns before making any well-founded scientific statements.’
Stem cells deemed safe to be used
This is the first time that stem cells have been administered via nasal drops to newborns with brain damage. ‘We closely monitored these ten newborns,’ Benders says, ‘and there were no infections or new formations found in the follow-up MRI scan, which indicates that this is a safe method, as we also demonstrated earlier in animal studies. For us, this is a significant step in the development of a new therapy for children who, until now, had no available treatment.’
Benders and Nijboer conducted this study successfully in a close collaboration between the lab and the clinical department, including researchers Nienke Wagenaar, Niek van der Aa and Lisanne Baak, the expertise of the cell therapy facility at the UMC Utrecht, and other NICUs (Neonatal Intensive Care Units) in the Netherlands. Without the help of the other hospitals, this study would not have been possible. They transferred patients to the Wilhelmina Children’s Hospital in Utrecht specifically for the treatment, demonstrating the feasibility of stem cell therapy within one week of symptoms appearing.
“When a cerebral infarction goes unnoticed, the motor and cognitive consequences become visible only after about a year. Then the neuroplasticity of the baby’s brain can no longer be optimally utilized.”
Onwards to larger clinical trials
Regarding further research, the doctors and researchers are aware that they asked parents to participate at a difficult time. Benders: ‘In these ten newborns, brain damage was detected at an early stage. Of course, that is very difficult, sad, and impactful for them. However, we can provide these parents with information and intensive guidance.’
‘When a cerebral infarction goes unnoticed, the motor and cognitive consequences become visible only after about a year,’ she adds. ‘Then the neuroplasticity of the baby’s brain can no longer be optimally utilized.’ Cora Nijboer and Manon Benders are now focusing on the next steps in their research, with an emphasis on large-scale placebo-controlled trials to further study the therapeutic effects of stem cells. Simultanously, we are exploring whether other neonatal patient groups with brain injury might benefit from stem cell therapy.
Read more about the work of Nijboer, Benders and colleagues.

Towards a new treatment of damaged cartilage
In a different department of the UMC Utrecht, orthopedic surgeon Roel Custers is further advanced in the process of bringing a new stem cell therapy to patients. Custers is running a study aiming to make an innovative knee cartilage treatment available for regular medical care. Supported by funding from ZonMW, this project seeks to improve the approach to cartilage damage in the knee.
Roel Custers has been working on a better treatment for knee cartilage damage, known as the IMPACT treatment. Throughout the IMPACT studies, Custers explores the use of donated stem cells to stimulate knee recovery. One key advantage of the IMPACT treatment is that it requires only one surgery, in contrast to the current approach that involves culturing the patient’s own stem cells before re-implantation. As a result, the IMPACT treatment is both faster and more cost-effective.
Improving the current standard of care
At present, the leading treatment involves cell therapy (often combined with osteotomy). In this process, a small amount of healthy cartilage tissue is extracted from the knee, and the cells are cultivated before being re-introduced about six weeks later. While effective, this approach has significant drawbacks, including the need for two surgeries, increasing the risk of complications, and putting additional strain on operating rooms and healthcare providers. Moreover, patients experience immobility during the six-week interval, and the procedure is costly.
Roel Custers: ‘What I am attempting to do now is to combine everything into one treatment. We still obtain that cartilage tissue, but we mix it with an adequate amount of donated stem cells and reimplant them during the same operation.’
“What I am attempting to do now is to combine everything into one treatment. We still obtain that cartilage tissue, but we mix it with an adequate amount of donated stem cells and reimplant them during the same operation.”
IMPACT is making impact
The progress made so far with the IMPACT treatment has been significant. The final clinical trial, IMPACT-2, is currently ongoing – all participants have been recruited, with results expected to be available next year. Additionally, funding from Reuma Nederland and ZonMW supports the investigation of the necessary steps to implement this groundbreaking treatment as the standard care for patients with cartilage damage.
Overcoming the regulatory hurdles
Despite the almost complete medical evidence for the IMPACT treatment, gaining approval for such innovative treatments within the healthcare system poses challenges. Custers explains that this is not a patient-involved study but rather focuses on overcoming regulatory obstacles. ‘The IMPACT treatment falls under the category of ATMP (Advanced Therapy Medicinal Product), an innovative treatment class with only a few approvals in Europe. Waiting for the study to be fully concluded before considering regular care access is impractical due to the lengthy processes involved.’
ATMPs often lack substantial industry backing, making the funding provided by ZonMW invaluable in carrying out the necessary investigations and overcoming regulatory hurdles. Custers: ‘What I would like is for us not only to continue treating patients here but also to enable other hospitals to do the same. However, this requires approval, for example, for the preparation of stem cells in the operating room.’ With the IMPACT study’s innovative approach and the support of organizations like ZonMW, Roel Custers aims to advance knee cartilage treatment, benefiting patients, healthcare providers, and the overall healthcare system.