How do you improve the quality of life for kidney patients? Worldwide, 3.9 million patients depend on renal replacement therapy. Those who are not eligible for a transplant, or waiting for one, are dependent on dialysis. Although this is life-saving, it also has disadvantages. Will kidney patients receive an implantable artificial kidney in the future?
Dialysis has been saving lives for eighty years…
More than 1.7 million Dutch people have some degree of kidney damage. Kidney damage means that your kidneys can no longer effectively rid your blood of waste products and can no longer properly regulate the salt and water balance. If that damage becomes extensive, one can develop complaints and at some point renal replacement therapy will be necessary. That concerns around 18,000 Dutch citizens. Their best option is a kidney transplant but not everyone is a suitable donor/recipient and there is a waiting list. A large proportion of patients with renal failure is therefore (temporarily) dependent on dialysis. Dialysis is a treatment that filters the blood of the kidney patient to remove waste products and restore the salt and water balance.
…but there are major drawbacks
Dialysis is an important technique that has saved many lives, but nephrologist and clinical researcher Karin Gerritsen explains that it is time to improve it. “A lot has changed in the past 50 years. We used to have huge landline phones, but now we have a smartphone that can do much more than just call. But little progress has been made in dialysis. For example, the dialysis machine is still a huge human-sized device that patients cannot take with them.”
“I think it is extremely important to improve the quality of life for patients and to make dialysis treatment more sustainable.”
Dialysis is ready for an upgrade
There are several directions in which dialysis can be improved. “Firstly, the blood purification with the current dialysis is not good enough,” Gerritsen begins, “as a result of which patients get all kinds of problems due to waste products that remain in the blood. In addition, a dialysis treatment requires an enormous amount of water and energy. That is not good for the mobility of the patient who sometimes wants to go on holiday, but it is also not good for sustainability. I think it is extremely important to change that, to improve the quality of life for patients and to make dialysis treatment more sustainable.”
First a portable dialysis machine
There are therefore three points for improvement: the dialysis device must become smaller, better at purifying and more sustainable. UMC Utrecht is working on this together with various partners. Gerritsen: “Reducing size is the first challenge. Dialysis requires a lot of flushing fluid to purify the blood. At the initiative of the Kidney Foundation, a new dialysis device has been developed in recent years, the NeoKidney from NextKidney. In this, the rinsing fluid is continuously cleaned during the treatment so that you can reuse it.”
The reuse limits the amount of water needed (4.5 liters instead of 120 liters per treatment), making the device smaller and portable. In 2024 this device will be tested in patients. Gerritsen’s group is now working on technologies to make the device even smaller. Gerritsen: “The small device gives patients the opportunity to dialyze more easily, more flexibly and more often, which can contribute to a better quality of life for the patient.”
“Reducing size is the first challenge. Dialysis requires a lot of flushing fluid to purify the blood. At the initiative of the Kidney Foundation, a new dialysis device has been developed in recent years, the NeoKidney from NextKidney. In this, the rinsing fluid is continuously cleaned during the treatment so that you can reuse it.”
Then, also for peritoneal dialysis
The NeoKidney is a hemodialysis device. In hemodialysis, the blood is purified by a stand-alone machine. There is also another form of dialysis: peritoneal dialysis. With the aid of a catheter in the abdominal wall, rinsing fluid is introduced into the abdominal cavity to purify blood internally. Reusing this dialysate, as is done in the NeoKidney, can also be useful for this other form of dialysis. With peritoneal dialysis, one usually has to replace the dialysate four to five times a day. Gerritsen: “We have now developed a portable device within the CORDIAL project, that uses only one dialysate filling per day and continuously regenerates this dialysate while the blood is purified better. We are currently testing this device in patients.”
Next up is better purification
To improve the purification of the blood, Gerritsen, and colleagues from UMC Utrecht but also Utrecht University, UTwente and IMEC, try to better imitate the natural filtering function of the kidney. “Materials that we are currently investigating are adsorbing membranes and silicon membranes. Silicon membranes can be made to resemble the membrane of our own kidney filter, with high permeability and selectivity. We also conduct research into increasing the purification of protein-bound waste that is difficult to remove. We will see if we can better remove these substances with the help of electromagnetic fields, in project MI-TRAM, or if we can use real kidney cells to do this, in project BAKtotheFuture.”
Towards an implantable artificial kidney
With better filtration and less water consumption, can we make a mobile artificial kidney that you can carry with you, or even have it implanted? Within the project KIDNEW Gerritsen is working on the first steps towards such an implantable artificial kidney, using a combination of non-biological materials and cells. “My fellow nephrologists Marianne Verhaar and Maarten Rookmaaker are working on the development of a fully biological kidney. I myself am concerned with the step before that, where a part is still non-organic,” says Gerritsen.
Harnessing the power of organoids
Since dialysis is renal replacement therapy, it could in principle be aided or improved by using healthy kidney cells. Organoids, the Utrecht invention that is slowly changing the field of disease models and regenerative therapies, could play a role in this.
The power of the kidney
The kidney consists of functional parts called nephrons – about a million of them. These nephrons consist of a ‘glomerulus’, a filter with a bundle of capillary vessels through which the blood undergoes an initial coarse filtration, and a ‘tubule’ or renal tube through which the preliminary urine formed in the glomeruli then flows. The renal tubules are an important part of the kidney: they remove the most difficult waste products from the blood, and they reabsorb useful substances from the pre-urine, so that you do not excrete it. In addition, they make hormones such as erythropoietin. Dialysis mainly mimics the glomerulus, which means that as a patient you miss the important, refined functions of the renal tubule.

A mini version of an organ
At UMC Utrecht and Hubrecht Institute, Fjodor Yousef Yengej studies miniature versions of the renal tubule. “I work with cultured mini-organs called organoids,” says Yousef Yengej. Organoids were discovered in 2009. They are very small clumps of tissue that resemble the organ they are made of. The first organoids were made from intestines. Our intestines contain many stem cells that continuously divide to renew the intestinal mucosa.
The daughter cells, which are created by division, specialize into cells that carry out the function of the intestine, for example the absorption of nutrients. To make intestinal organoids, you can take some tissue from the intestine, containing the stem cells. If you add the right growth substances, these stem cells will divide and produce daughter cells with different functions, just like in the body. This creates a small piece of tissue that resembles the intestine in terms of composition, and mimics some of the functions of the intestine.’
Renal organoids offer opportunities for research
“Organoids offer fantastic opportunities for research, and since 2019 we can also make organoids from stem cells from the kidneys,” says Yousef Yengej. Moreover, it has even been possible to make kidney organoids from cells from the urine. As a result, they can also be cultured from the large group of patients who do not require surgery or a biopsy, and even from healthy individuals. The kidney organoids can then be cultured fairly easily and used for all kinds of research.
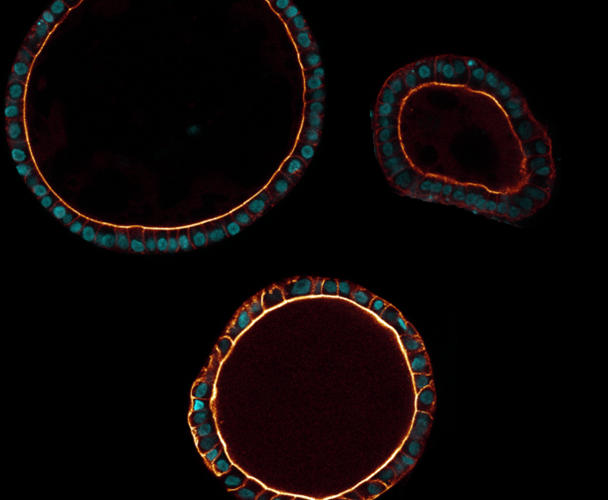
Yousef Yengej: ‘It turns out that the renal organoids mainly consist of the inner lining of the renal tubes (tubules), which is why we call them ‘tubuloids’. That is precisely the part of the kidney where most functions take place, and therefore where most diseases occur. In other words, we find this part of the kidney the most interesting for research.’
A look inside the kidney
The tubuloids hold promise for research for several reasons. “First, they provide a window into the healthy or diseased kidney,” says Yousef Yengej, “because these are pieces of tissue whose cells are identical to those of the donor, be it a healthy person or someone with kidney disease. We don’t have to artificially immortalize or reprogram the cells to study them, and see the same things happen as in the real kidney.’
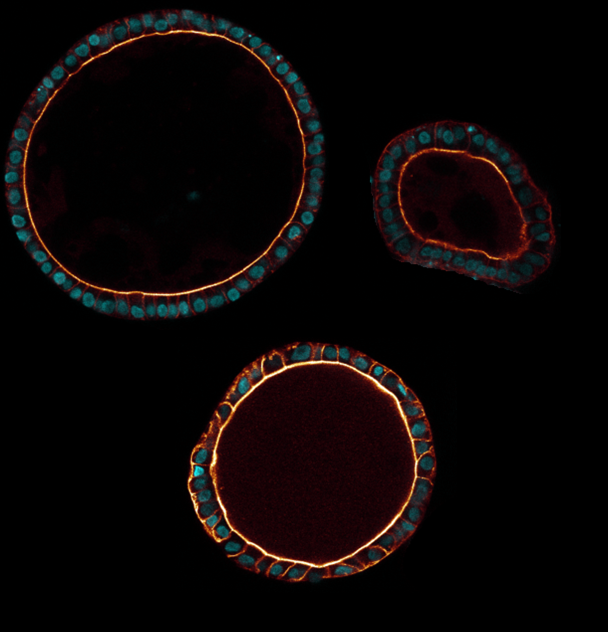
Tubuloids
When the tubuloids are derived from a healthy person, the organoid tissue behaves as it should. Because there is still so much to learn about exactly how the kidney works, and how all the different processes that excrete or absorb substances take place, research with healthy tubuloids is yielding a great deal of knowledge. On the other hand, it’s also possible to grow tubuloids from tissue of patients with, for example, a hereditary kidney disease or kidney cancer, or expose healthy tubuloids to viruses or harmful substances. That way we can study how this affects the tissue, which in turn can provide a lot of valuable information about kidney disease.
Using organoids to test treatments
“But there is much more potential in the tubuloids of kidney patients,” says Yousef Yengej. ‘For example, it may become possible to grow a larger quantity of those organoids and then test which medicines work best for a patient. We have now done this for various disorders, for example by giving organoids that we made from kidney tumor cells different types of chemotherapy. Those results are promising, although we are not yet ready to apply them in the clinic.’
Can organoids also supplement or replace dialysis?
So it seems that tubuloids could play a role in the future in the clinic, in the testing and discovery of new drugs for kidney disease. Yet, Yousef Yengej is also working on a bigger leap, and that is applying the tubuloids to replace damaged kidney tissue by using them in dialysis.
“We hope that we can use the cultured renal tubules to improve dialysis. Dialysis removes excess fluid and water-soluble (waste) substances but does not replace the many sophisticated functions that take place in the renal tubule. As a result, for example, useful nutrients are also lost, while large and protein-bound waste products – which do not fit through the filter – are not properly excreted. Hormonal functions, such as activation of vitamin D, are also not simulated. Tubuloids have the potential to perform precisely these functions, making kidney replacement much better.’
How to enhance dialysis with organoids
But how should that work? ‘In the beginning, we may be able to combine the functionality of the tubuloids with the dialysis device. But who knows, in the long term we may even be able to turn it into something that can be implanted and thus take over the kidney function in the body.’
Such a bio-artificial kidney is still far from practice, but distant views motivate Yousef Yengej in his work. ‘Another option is that we use the restorative capacities of the tubuloids to locally repair the renal tubules if they are damaged. Perhaps one day we may even be able to build a larger, working part of the kidney by combining tubuloids with body-friendly materials and possibly other cells. Then we can actually give the patient back a piece of kidney made from the body’s own material, without the immune reactions that are now associated with a donor kidney.’