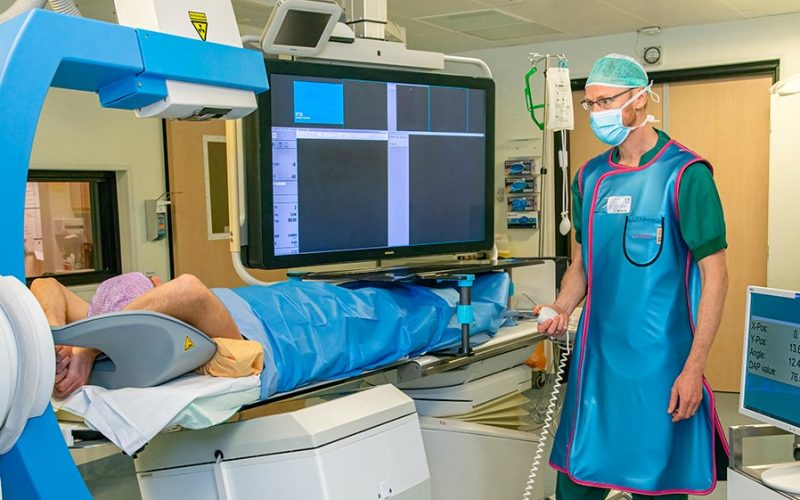
Treated in two hours instead of two weeks: the go-ahead has been given for two clinical trials in which liver cancer patients will actually be treated with the hybrid C-arm. The device, an invention of UMC Utrecht, speeds up and eases radioembolization. This project is part of the IMAGIO consortium, which has now received an IHI grant of no less than 24 million euros from the European Union and its partners.
Radioembolization is a form of internal radiotherapy to treat patients with liver cancer or metastases in the liver. The treatment is given to virtually out-of-treatment patients. For instance, people who can no longer have surgery, or patients who no longer respond or are experiencing too many side-effects to chemotherapy. Under local anaesthesia and through the groin or wrist, the radiologist injects radioactive beads into the hepatic artery. Normally, the procedure (with the ‘traditional’ C-arm) takes one to two weeks, for which the patient must visit the hospital twice. With the new, hybrid C-arm, the patient completes the treatment in just one session of about two hours.
UMC Utrecht’s Hugo de Jong (professor of Medical Physics Radiology and Nuclear Medicine) has invented the renewed, hybrid C-arm. “As clinical physicists, we are constantly involved in devising new therapies. Surgery was the old standard way to treat patients, which is of course very invasive. Now, we prefer less invasive therapies, for which we only have to make one incision or no incision at all,” Hugo explains. But then, of course, you need good equipment to look inside the patient and monitor the operation. Hugo continues: “For years, we have been developing and researching such devices here, for example in the fields of MRI (such as the well-known MR-Linac), CT, X-ray and ultrasound.”
Professor Hugo de Jong
The C-arm works with X-rays. During radioembolization, it helps the radiologist to properly visualise the blood vessels and tissue around the hepatic artery. Hugo: “Years ago, I suddenly realised: radioembolization could be much less inconvenient for the patient if the C-arm could perform several functions at once. Because it is an intensive procedure now, especially when you consider that patients are often very ill.”
Why does radioembolization normally take so long? That’s because a trial procedure must be carried out first, to make sure that the beads enter the liver properly and safely. “For example, they should not end up in the stomach or lungs, which could cause serious damage”, Hugo explains. During treatment, the patient lies under the C-arm. A catheter is inserted through a small incision in the groin or wrist. The X-ray allow the radiologist to do his work precisely. Branches of the hepatic artery that do not go directly to the liver are closed off. And to test whether one safe pathway leads to the liver via the tube, a low dose of radioactive beads is injected, mimicking the actual treatment.
After the test procedure in the morning, the incision is closed. Then, in the afternoon, the patient goes to the nuclear department for a SPECT scan. Are all branches of the hepatic artery properly closed? Did no beads end up outside the liver? Did they travel to the right location? If the trial treatment has been carried out safely, the patient will return in about two weeks for the actual procedure. Then, another incision is made, and the whole procedure starts all over again, but now with a higher dose of radiation in the beads.
The hybrid C-arm will impact this two-week treatment process considerably, because it combines the work of the traditional C-arm with the SPECT scan. This will ease the treatment for the patient in a major way, with less uncertainty and stress. Plus: the incision will only have to be made once, and the patient will stay at one location during the entire procedure. Hugo: “We will also avoid the risk that the tumour will have evolved in a fortnight. That is, when patients are this ill, their tumours tend to change quickly.” Furthermore, with the new device, the radiologist can use clearer, live images. “This allows him/her to make continuous adjustments. For example, he/she can immediately see whether he/she needs to reposition the catheter, or administer more beads.”
About ten years ago, Hugo and his colleagues started building several prototypes of a new C-arm. With one of these, they eventually continued their research. Recently, the hybrid C-arm was tested for safety during a patient study. Now, thanks to the new IMAGIO-grant, the next step can be taken: during two clinical studies (with 12 patients each) the actual treatment will be carried out from A to Z. Hugo will work together with fellow applicants Maarten Smits (interventional radiologist), Marnix Lam (nuclear medicine physician) and Martijn Dietze (researcher).
But, before the actual trials start, the device’s software and hardware will first be slightly modified. And TU Delft (Delft University of Technology) will help in the future to make the device more compact so that it can be more easily moved and/or integrated into already existing devices.
“The whole project will take four years. We hope to actually start treating patients in a year, at best. Preparing the clinical trials is a careful and extensive process, which we are still working on. For this study, we need to look carefully at which patients would best qualify for the trials”, Hugo says.
Meanwhile, the hybrid C-arm may already offer prospects for other conditions. Hugo: “The device could also improve cardiac catheterisation, for instance. We’ve recently received another grant to start tentatively working out that idea.”
Within the IMAGIO consortium (‘Imaging and advanced guidance for workflow optimisation in interventional oncology’), some 30 public and private parties work together on less invasive, image-guided therapies with lung cancer, liver cancer and soft tissue tumours (sarcomas). Besides UMC Utrecht and TU Delft, for example, LUMC, MUMC+ and Radboudumc are also working on IMAGIO projects. IMAGIO has now received a total grant of €24 million euros from Innovative Health Initiative, a public-private partnership between the European Union and European pharmaceutical partners.
Read more about the grant and the IMAGIO consortium in this press release.
Read more in this news article about the five projects, including IMAGIO, which are supported by IHI.
The clinical trial with the hybrid C-arm has not started yet. Potentially suitable patients will be identified during a multidisciplinary consultation. Patients will then be approached by their own treating physician, asking them if they would like to participate. When the clinical trial will start is not known yet; it will probably take another one to two years.
This project is supported by the Innovative Health Initiative Joint Undertaking (JU) under grant agreement no. 101112053. The JU receives support from the European Union’s Horizon Europe research and innovation programme and life science industries represented by COCIR, EFPIA, EuropaBio, MedTech Europe and Vaccines Europe.
Disclaimer: IMAGIO is funded by the European Union, the private members, and those contributing partners of the IHI JU. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the aforementioned parties. Neither of the aforementioned parties can be held responsible for them.