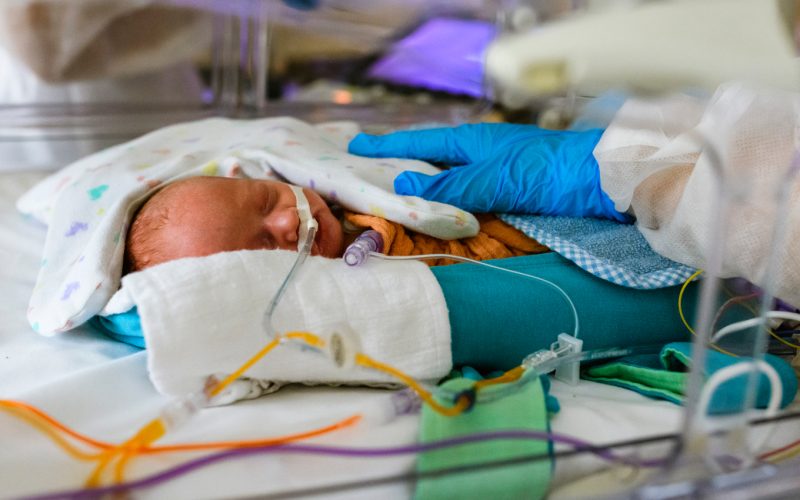
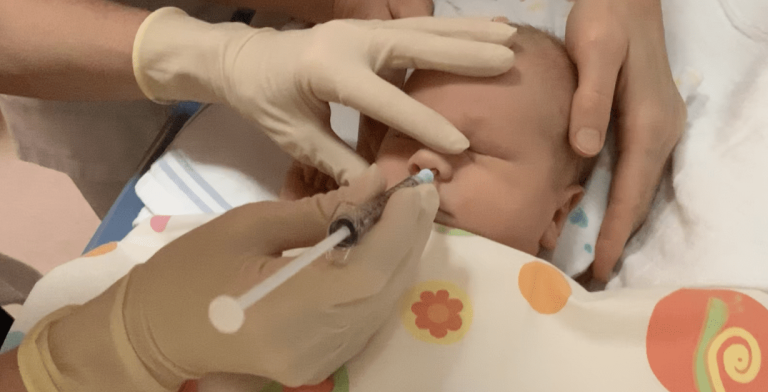
Oxygen deprivation around birth can lead to brain damage in babies, with far-reaching consequences. A new stem cell treatment administered via nasal drops is showing promising results. In a safety study conducted at UMC Utrecht, called PASSIoN, ten newborns received this ‘intranasal stem cell therapy’ shortly after birth. Most of the children showed remarkably positive development: they started walking earlier on average than untreated children with comparable brain damage, had no motor impairments, and none developed epilepsy or visual problems. The study results were published today in the scientific journal Stroke.
All ten babies in the study had a perinatal stroke: a type of brain injury that occurs just before, during, or shortly after birth, damaging the developing brain. This kind of injury can lead to long-term neurological problems such as cerebral palsy (CP), a condition that affects movement due to early brain damage.
Support this research with your donation.
Every donation counts. Donate now.
In the study, the ten babies received a single dose of mesenchymal stem cells, administered as nasal drops within a week of birth. Two years after treatment, none of the children experienced side effects. There were two hospital admissions, but these were unrelated to the therapy. None of the children required medication after being discharged from the hospital.
The amount of brain tissue loss was also smaller than expected, given the severity of the strokes. Most children developed well. One child had a mild cognitive delay, two had language delays, and one suffered from severe sleep problems.
Motor development also proved surprisingly positive. Only two children developed mild cerebral palsy. That’s 20% of the treated group, compared to 50% in a historical control group of children with a similar type of stroke. Scientific literature even reports rates of up to 70%.
Interestingly, all children in this study initially showed damage to the brain’s motor pathways – something that typically brings a CP risk of over 80%, depending on the size and exact location of the infarct. None of the children developed epilepsy or vision problems.
“Seeing such positive development in a high-risk group like this is truly extraordinary,” says pediatrician and professor Manon Benders. “It gives not only the parents but also us as a medical team real hope.”
It is important to note that the PASSIoN study was not designed to prove the effectiveness of the stem cell treatment, but to assess its safety. To definitively determine whether stem cell therapy works, a new study — the iSTOP-CP study — is expected to launch in early 2026.
“We’ve spent years conducting fundamental research in the lab, where we saw that stem cells have enormous potential for brain repair,” says neuroscientist and professor Cora Nijboer. “And we continue to develop and optimize the treatment in close collaboration with researchers in Maastricht. But these results, in such vulnerable babies, are exactly what we’ve been working toward. We’re incredibly proud and excited about the start of the iSTOP-CP study. Hopefully these promising outcomes will hold up – or even improve – in the coming years.”
Cora Nijboer and Manon Benders
A total of 162 newborns with brain damage due to stroke or severe oxygen deprivation will participate in the upcoming study. Within seven days of birth, they will receive either stem cells or a placebo. Researchers will then closely monitor their development up to the age of 24 months. If the therapy proves effective, it could significantly change how brain injury in newborns is treated.
Health economist Renske ten Ham, also a researcher at UMC Utrecht, will carry out a cost-effectiveness analysis during the study. She will evaluate how the costs and benefits of this promising new treatment compare to current care, including the impact on the child’s development and the burden on parents.
Manon: “The coming years will be very exciting, but we are confident that this study will mark an important step forward for children with brain injury.”
Read the scientific articleThe iSTOP-CP study is receiving broad support from both national and international patient organizations, including CP Nederland and Care4Neo in the Netherlands, the Cerebral Palsy Foundation in the US, Cerebral Palsy Alliance in Australia, and Fondation Paralysie Cérébrale in France. Due to the rarity of this brain condition, collaboration is essential. All neonatal intensive care units in the Neonatology Network Netherlands (N3) are therefore participating in the study.
The Dutch Healthcare Institute’s ‘Promising Care’ subsidy scheme helps make this new and potentially effective treatment available to patients more quickly. Because research into such treatments is often expensive, the scheme covers the high care and research costs. If the new study shows that the stem cell treatment works, it could be included in the basic Dutch health insurance package. In this way, the Healthcare Institute and ZonMw are helping to improve the quality and faster accessibility of promising new care.