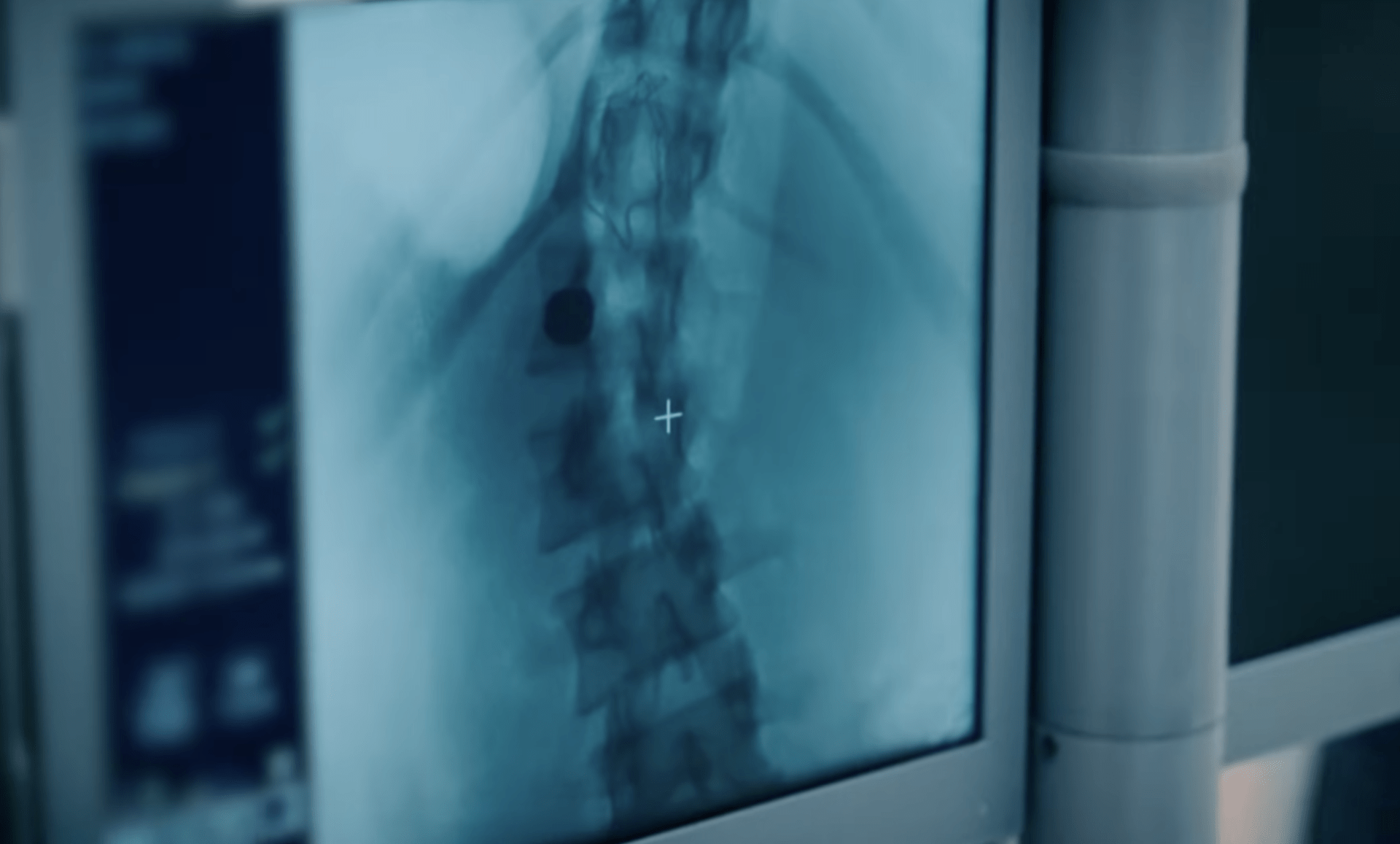
Children with severe scoliosis often need multiple major spinal surgeries as they grow. The Spring Distraction System takes a different approach: the implant supports the spine while the child grows, without fixating it. The American Food and Drug Administration (FDA) has now approved the implant and granted it the breakthrough device designation. This allows the system to be used more quickly in American hospitals. “This is just the beginning,” says Moyo Kruyt, developer and orthopedic surgeon at the UMC Utrecht.
In scoliosis, the spine is severely curved. The current standard treatment often involves fixating the spine with metal rods. While this stops the curve from progressing, it also limits the child’s growth. Children often need a new surgery every six months, which is very demanding for both them and their families.
The Spring Distraction System works differently. The implant is a spring-driven device that continuously applies gentle force to the spine, helping it grow straighter over time. The spring grows along with the child and does not need repeated surgical adjustments.
“We encourage the spine to heal itself,” explains Moyo Kruyt, who developed the implant and treats scoliosis patients on a daily basis. “This mainly starts in the intervertebral discs. They regain a more normal shape and elasticity. It’s a completely different way of treating scoliosis.”
The Spring Distraction System is developed by the start-up Cresco Spine, where Moyo works closely with a team of entrepreneurs and orthopedic surgeons, including his colleague and co-inventor Rene Castelein.
The FDA granted the implant a breakthrough device designation. This status is not given lightly. It recognizes medical innovations that offer such a significant benefit to patients that they should be made available as soon as possible.
According to Moyo, the recognition is important: “This is a major milestone for us. The Spring Distraction System is actually a simpler version of what we envisioned ten years ago. This step brings us closer to even better treatments.”
Although the implant is approved, it cannot be used immediately. The operation is specialized and requires extensive experience. That is why the team is now training doctors in leading clinics in the United States and Canada.
The developers hope that the American approval will also help with European authorization, which is more complex due to strict regulations.
“The fact that the implant can now be used in the U.S. is an important step,” says Moyo. “It provides knowledge, experience, and funding, which will eventually allow us to help children in Europe as well.”
The ultimate goal goes beyond this single implant. Moyo and his team at Cresco Spine are working on a series of new dynamic implants that can correct the spine in multiple directions, allow natural movement, and promote regeneration. “These three-dimensional implants not only restore the spine’s length, but also address rotation and sideways curvature,” Moyo explains.
Animal studies have already shown promising results. The next step is a clinical trial with a small group of patients. For this, the team is collaborating with European partners and requires additional funding.
“Our goal is to create a new way of treating scoliosis that focuses on healing the spine, rather than fixating it,” he says. “What we do in clinics today is quite outdated. This can be improved.”