No. The NeoKidney is currently only used within clinical research.
Patients with severe kidney failure are often dependent on dialysis in the hospital. At UMC Utrecht, patients are now testing a portable artificial kidney for the first time in the Netherlands. Patients can use it at home. The first patients are participating in a clinical study with the so-called NeoKidney. The goal: more freedom and control in daily life, without compromising safety.
Healthy kidneys filter waste products and excess fluid from the blood. In kidney failure, the kidneys function insufficiently or not at all. To survive, a treatment is needed that replaces kidney function. A kidney transplant is usually the best option. However, many patients are on the waiting list or cannot receive a transplant, so they must rely on dialysis to stay alive. Hemodialysis typically takes four hours each time, and often three times per week.
In hemodialysis, the blood is purified several times per week via a machine. This often takes place in the hospital and takes several hours per treatment. This has a major impact on work, study, travel and social contacts for many people. In the Netherlands alone, this involves about 5,000 people; worldwide, about 2.8 million people depend on hemodialysis. In total, about 6,200 people undergo dialysis in the Netherlands. Worldwide, 3.8 million kidney patients depend on dialysis to survive.
“Dialysis is life-saving, but also very burdensome,” says Karin Gerritsen, nephrologist at UMC Utrecht and principal investigator of the study. “With a portable artificial kidney, we want to give patients more freedom and make the treatment fit better with their daily lives.”
A portable artificial kidney makes it easier to:
This can contribute to a better quality of life.
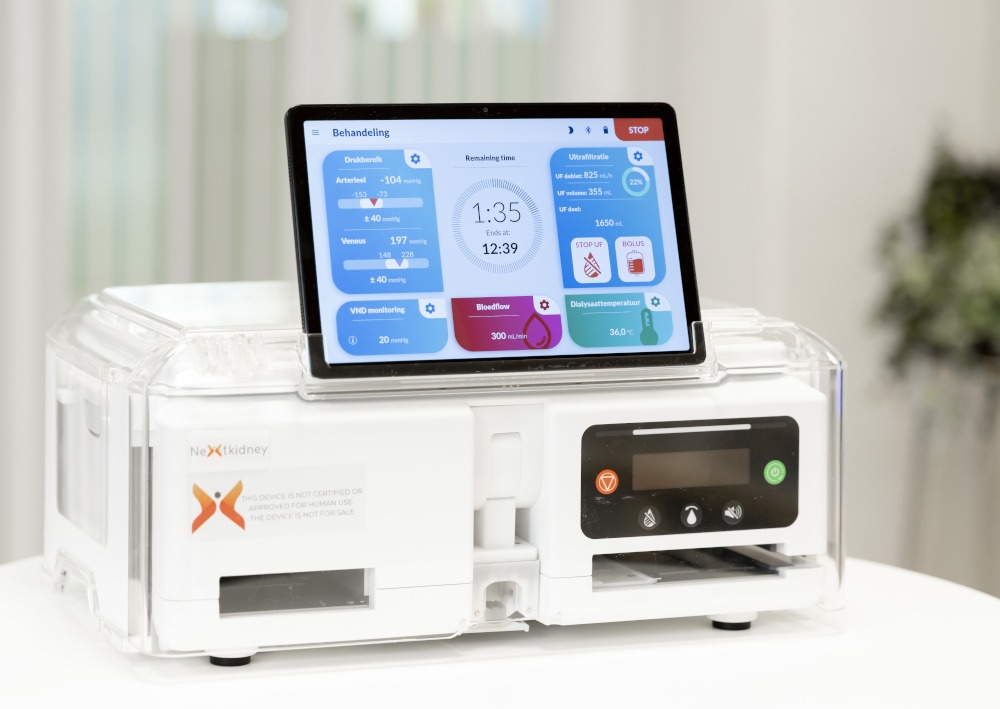
The NeoKidney is a lightweight, portable hemodialysis device (approximately a carry-on rolling suitcase). The device is developed for home use, but in a much more compact form than current hemodialysis devices.
A Neokidney portable dialysis device
Unlike standard (home) hemodialysis, no home modifications are needed, such as a connection to water supply and drainage, an extra electrical group, or (medical) grounding. The device is energy-efficient and electrically safe, so it can be connected to a standard power outlet.
Important features:
The device is designed for short, frequent hemodialysis, 4 to 7 treatments of 2 hours per week. This allows the NeoKidney to offer a more gradual treatment than the traditional schedule of three times per week four hours, with fewer fluctuations in fluid, waste products and minerals. The device is connected in the same way as regular hemodialysis, via a shunt or a catheter.
UMC Utrecht, part of the Utrecht Science Park and affiliated with Utrecht University, is conducting research together with the Kidney Foundation and medical technology company NextKidney into the safety and effectiveness of the NeoKidney.
After earlier positive safety studies with earlier prototypes in France and Singapore:
Karin: “During this test phase, we are investigating whether the NeoKidney works well and safely. First of all, we investigate whether the device cleans the blood sufficiently. We do that by measuring during dialyses how effectively waste products are removed from the blood. We want to be sure that this happens at a reliable and consistent level. We keep track of how much fluid is removed and whether that is done accurately.”
“Additionally, safety is very important. We carefully monitor whether side effects occur during or after dialysis that are related to the device, and whether they are serious. We also follow changes in, for example, blood pressure, heart rate and blood values to check whether the body responds well to the treatment,” says Karin.
“Furthermore, we ask patients themselves how they experience the treatment and what the effect is on their daily life and quality of life. Halfway through the study, NextKidney will submit the results to an independent certification organization. This organization assesses whether the device is safe and works well enough to be allowed on the market.” The goal is to work towards CE certification, possibly in 2026 or 2027. This is necessary before the device can become available to patients in Europe.
The development of the portable artificial kidney is the result of close collaboration between:
Karin Gerritsen, nephrologist UMC Utrecht, and Tom Oostrom, director Kidney Foundation
“By combining knowledge and experience, we can work step by step towards better kidney care,” says Karin Gerritsen.
The portable artificial kidney is an intermediate step towards further innovation. Researchers are also working on a future vision in which dialysis becomes possible without large devices or needles, for example with implantable technology. This falls within the broader field of regenerative medicine. Regenerative medicine develops new treatments in which the body’s self-healing capacity is used.
“That is still future music,” Gerritsen emphasizes. “But every step brings us closer.”
If the research is successful, the portable artificial kidney can in the future:
The research is still ongoing. Results will be announced in 2027.
Please contact your own treating physician/nephrologist. Your treating doctor/nephrologist can refer you to UMC Utrecht for participation.
You can also contact our nephrology secretariat:
No. The NeoKidney is currently only used within clinical research.
Safety is being extensively investigated. Earlier studies were positive, but further research is needed.
The portable artificial kidney is currently only available within a clinical study and is intended for patients with severe kidney failure who are receiving hemodialysis and are eligible to participate in the study. All patients who meet the inclusion and exclusion criteria may take part. Participation is possible via a referral by the treating physician/nephrologist.
That depends on the research results and CE certification, possibly from 2027.
No. Dialysis remains a treatment that replaces kidney function. A transplant is still preferred, if possible.