Macrophages actively control resolution of inflammatory pain remotely from the site of inflammation by transferring mitochondria to sensory neurons. Pain resolution and the transfer of mitochondria requires the expression of the CD200 receptor (CD200R) on macrophages and its ligand iSec1 on sensory neurons. These research findings from UMC Utrecht reveal a novel mechanism for active resolution of inflammatory pain.
One in five Dutch suffer from chronic pain. A large number of people with chronic pain have inflammatory conditions such as rheumatoid arthritis, osteoarthritis or inflammatory bowel disease. This type of chronic pain is very difficult to treat, leaving many without effective treatment. The current paradigm is that inflammatory pain resolves passively after termination of inflammation. Yet, in a substantial proportion of patients with inflammatory diseases, termination of inflammation does not stop pain which leads to chronic pain. To identify potential novel pain treatments, a deeper understanding of the mechanisms that normally resolve inflammatory pain is needed.
The research group of neuro-immunologist dr. Niels Eijkelkamp at the Center for Translational Immunology (CTI) at UMC Utrecht has been working for years to unravel the mechanisms of chronic inflammatory pain. In a new study they joined force with the group of Prof Linde Meyaard. Now published in Neuron, they report that macrophages, a specific immune cell, shuttle mitochondria to sensory neurons that innervate inflamed tissue and transmit the pain signals from that tissue. The shuttling of mitochondria helps to resolve pain.
Michiel van der Vlist, Ramin Raoof, Hanneke Willemen, Linde Meyaard, Niels Eijkelkamp
For a long time, scientist thought that macrophages are simple, pathogen-eating immune cells. However, scientists came to realize that macrophages have a far more extensive list of job duties. They have specialized functions across body tissues, help to repair damaged tissue, play a key role in regulating inflammation and pain, the latter scientists are just beginning to reveal.
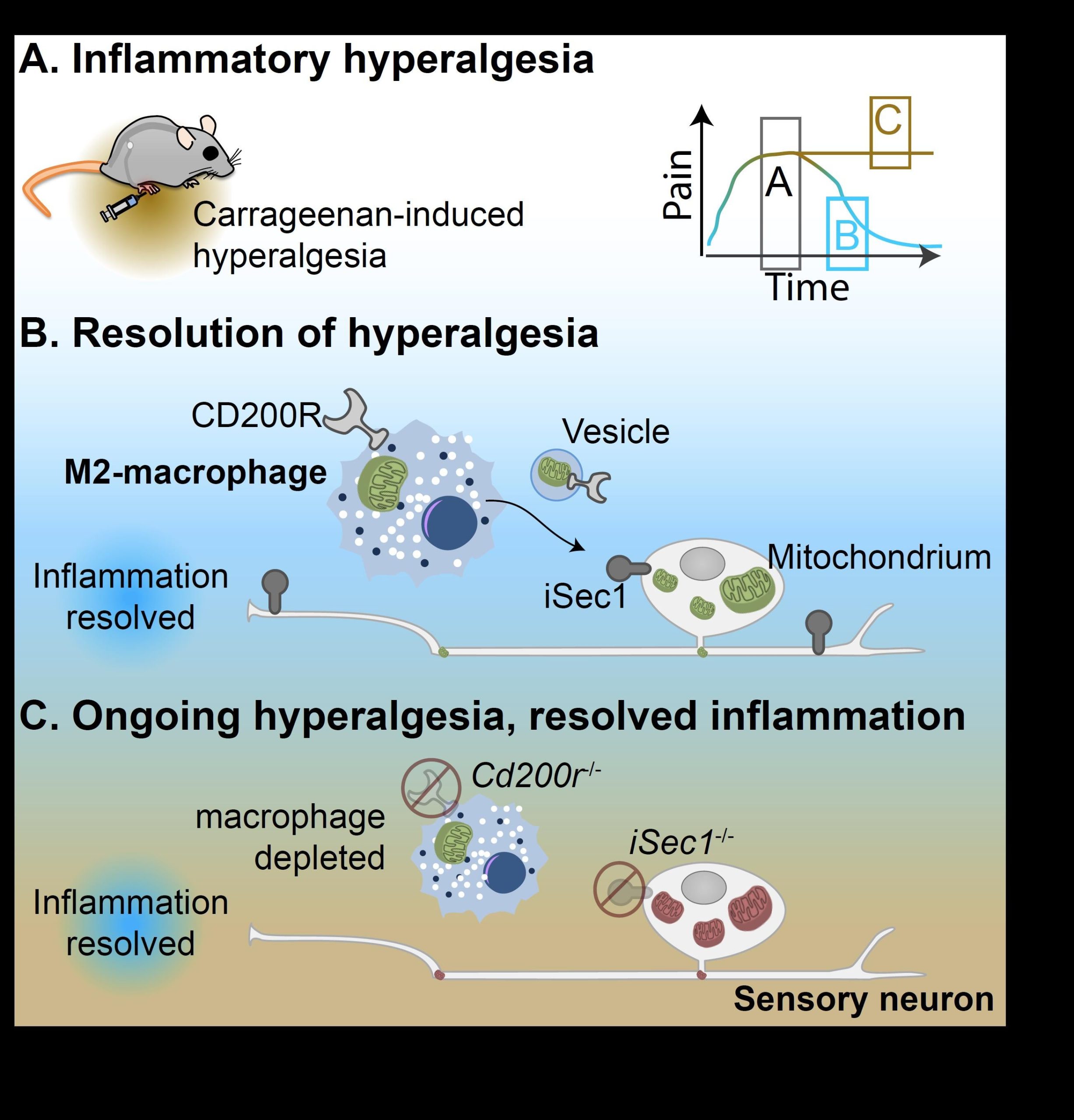
Dr. Ramin Raoof and dr. Hanneke Willemen in the Eijkelkamp group showed that macrophages actively control resolution of inflammatory pain in mice. During resolution of pain, macrophages increase in the dorsal root ganglia, the tissue that consist of the cell bodies of pain-transmitting neurons. At the same time that the number of macrophages increased in the dorsal root ganglia, the mitochondrial function of neurons improved and inflammatory pain resolved. In collaboration with prof. Linde Meyaard and dr. Michiel van der Vlist, they discovered that the resolution of inflammatory pain requires macrophages to release small vesicles that contain mitochondria, the powerhouses of cells. These vesicle shuttles the mitochondria from macrophages to the pain-transmitting neuron. They further found that to effectively shuttle the mitochondria, macrophages and their vesicles needed a specific receptor on their membrane: CD200 receptor (CD200R). The pain-transmitting neurons on the other hand needed to express iSec1, a ligand of CD200R. Similar to what sometimes happens in human inflammatory diseases, removal of CD200R, iSec1 or macrophages from mice caused failure of pain resolution that lead to persisting pain while inflammation did stop. These findings point to a model where CD200R and Isec1 serve as some kind of docking system for mitochondria-containing vesicles. These findings reveal a novel mechanism for active resolution of inflammatory pain.
Niels Eijkelkamp on ideas for follow-up research: “Our next step will be to demonstrate that replenishment of functional mitochondria in neurons during chronic inflammatory conditions actually improves mitochondrial function and that this is the reason for pain resolution. Moreover we will explore whether targeting mitochondria and/or macrophages is a way to promote pain resolution.”
Graphic abstract
Vlist M van der*, Raoof R*, Willemen HLDM*, Prado J, Versteeg S, Gil CM, Vos M, Lokhorst RE, Pasterkamp RJ, Kojima T, Karasuyama H, Khoury-Hanold W, Meyaard L, Eijkelkamp N. Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron 2021, in press.
* Shared first authorship