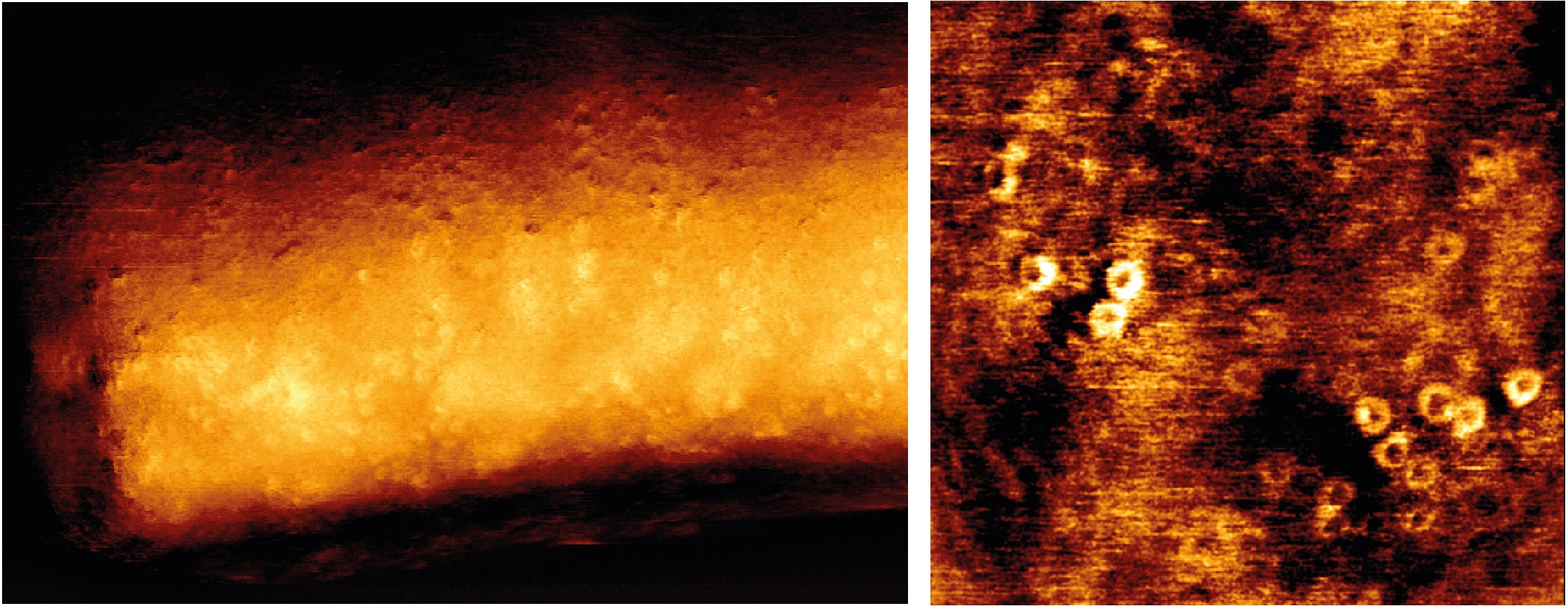
It is well-known that bacteria can be killed by specific proteins of the human immune system (called ‘complement’ proteins) that can create pores in the bacterial cell wall. PhD student Dennis Doorduijn from UMC Utrecht investigated, on a molecular level, how this pore formation takes place and how certain bacteria can evade this killing mechanism. He concludes that these insights provide interesting clues on how to develop new anti-bacterial therapies.
Bacteria that cause infections pose a great threat to global human health. Especially because some of these bacteria are becoming more and more resistant to current antibiotics, there is a huge need of alternatives to treat bacterial infections. So-called complement proteins of the human immune system can kill certain bacteria (Gram-negative bacteria) by creating ring-shaped pores (named the Membrane Attack Complex, MAC) in their cell wall. There is great interest in developing therapies that can direct these complement proteins to form MAC pores and kill bacteria. Therefore, it is important to understand in detail how complement proteins form pores. PhD candidate Dennis Doorduijn (Department of Medical Microbiology, UMC Utrecht) studied, on a molecular level, how complement forms pores in the bacterial cell wall.
In his thesis, Dennis Doorduijn and colleagues revealed which steps in the formation of MAC pores are crucial to kill many different Gram-negative bacteria. They showed that complement proteins have to be anchored to the bacterial cell surface immediately after they are activated to form a pore that can kill that bacteria. In addition, they found that MAC pores have to assemble completely to be able to kill bacteria. Certain Escherichia coli strains were able to prevent being killed by these pores. Although these “MAC-resistant” bacteria activate more complement proteins than bacteria that are sensitive to pores, these pores were not fully completed and not appropriately inserted into the cell wall. As a consequence, these pores are released from the cell wall, which then can result in pore formation and inflicting damage on neighboring cells. This damage to neighboring cells can be prevented by regulators in human blood, so further research is required to understand how relevant this effect on neighboring cells is in patients that suffer from bacterial infections.
Dennis Doorduijn on the findings in his PhD thesis: “Altogether, the research in my thesis helps us to better understand why some bacteria are killed by complement proteins and why some survive. It also suggests that some bacteria that are not killed by MAC pores can still potently activate complement proteins, which might result in damage to human cells. These considerations can be important in the future to decide if therapy that activates complement proteins can be used effectively and safely to treat bacterial infections.”
Pore formation in a bacterial cell wall
The complement system is a part of the immune system that enhances (complements) the ability of antibodies and phagocytic cells to: (1) clear microbes from an organism, (2) promote inflammation to attract phagocytes, and (3) attack the pathogen’s cell envelope with MAC pores. It consists of a number of proteins that are mainly synthesized by the liver, and circulate in the blood as inactive precursors. Complement proteins can be recruited to the surface of bacteria and brought into action by for instance antibodies that bind to bacteria. When complement proteins are activated on the bacterial surface, they form enzymes that convert other complement proteins that are released as cytokines and initiate an amplifying cascade of further conversion of complement proteins. The end result is stimulation of phagocytes to clear foreign and damaged material, inflammation to attract additional phagocytes, and formation of cell-killing MAC pores in the bacterial cell wall.
Dennis Doorduijn (1992, Delft) defended his PhD thesis on November 16, 2021 at Utrecht University. The title of his thesis is “How complement forms pores in bacterial membranes – a complex assembly”. Supervisor was prof. dr. Suzan Rooijakkers and co-supervisor was dr. Bart Bardoel (both Department of Medical Microbiology, UMC Utrecht). The studies in this thesis have been supported by an ERC Starting Grant.
Full text thesis: https://dspace.library.uu.nl/handle/1874/406706