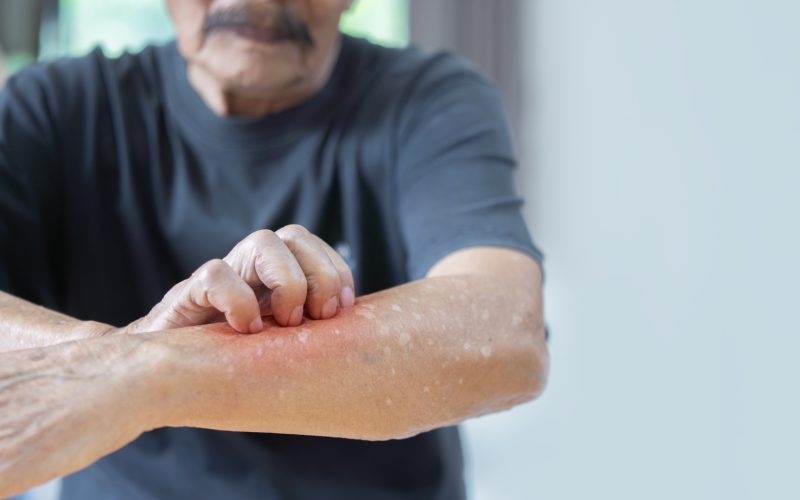
In the treatment of atopic dermatitis, the biologics dupilumab and tralokinumab offer targeted treatment, with real-world studies supporting safety, efficacy, and personalized dosing. Ongoing research explores immune mechanisms, ocular effects, and patient stratification to optimize long-term, precision-based AD management. These were the main take-aways of the PhD thesis of Coco Dekkers at UMC Utrecht.
The recent approval of biological medical products (‘biologics’) such as dupilumab and tralokinumab or the treatment of atopic dermatitis (AD) has revolutionized the treatment landscape for moderate-to-severe AD. Dupilumab targets the interleukin 4 (IL-4) receptor α-subunit, blocking the IL-4 and IL-13 signaling pathways, while tralokinumab specifically inhibits IL-13 signaling. Both therapies have demonstrated substantial effectiveness in clinical trials, but the real-world application and long-term outcomes remain a key focus of ongoing research.
Coco Dekkers, MD PhD
The Dutch BioDay Eczema and Atopic Diseases Registry, which launched in 2017, has been instrumental in collecting real-world data on the safety, efficacy, and patient satisfaction associated with these therapies. With over 2,000 participants, the registry has highlighted the importance of personalized treatment strategies for AD patients. One area of focus has been optimizing the dosing regimen for dupilumab.
PhD research by Coco Dekkers, MD (Department of Dermatology and Allergology, UMC Utrecht), with help of data from the BioDay registry, has shown that extending the dosing interval for patients with controlled AD (EASI ≤7) to every 4-8 weeks can maintain efficacy while reducing the risk of overtreatment and lowering healthcare costs. Tralokinumab, while newer than dupilumab, has also shown promising results in both clinical trials and daily practice.
Studies suggest that tralokinumab is effective for both biologic-naïve and biologic-experienced patients. Since the introduction of dupilumab as treatment for AD, dupilumab-associated ocular surface disease (OSD) has emerged as an important adverse event that requires close cooperation between dermatologists and ophthalmologists. Due to its more specific working mechanism compared to dupilumab, tralokinumab is hypothesized to cause less frequent and/or less severe OSD. Although OSD remains a concern for both biologics, the ongoing research at UMC Utrecht is exploring the effects of tralokinumab on ocular health, with promising findings suggesting a more favorable ocular safety profile for tralokinumab as compared to dupilumab.
Looking ahead, Dekkers envisions that the future of AD treatment will likely involve further optimization of biologic therapies, incorporating patient-specific factors such as immune profiles and treatment history. Stratification of patients based on their immune response could further refine treatment strategies, ensuring that each patient receives the most appropriate therapy. As the therapeutic pipeline expands, the challenge will be to balance clinical efficacy with safety, long-term outcomes, and cost-effectiveness.
Coco Dekkers concludes: “While current biologic therapies for AD represent a significant advancement, continued clinical and translational research is crucial to ensure optimal treatment strategies for all patients. With an increasing focus on precision medicine, the future of AD treatment holds the promise of more personalized, effective interventions, ultimately improving the quality of life for those living with this chronic condition.”
AD is a common chronic inflammatory skin disease, with a prevalence of up to 30 percent in children and up to 10 percent in adults. Clinically, AD is characterized by itchy, red, swollen and ‘cracked’ skin. AD often manifests early in life and can persist into adulthood, substantially impairing the quality of life due to persistent symptoms, sleep disturbances, and the psychological impact of visible skin lesions. The pathogenesis of AD is complex and multifactorial, including both genetic and environmental factors. According to the RIVM, nearly 400,000 people in the Netherlands have AD with a health care cost of approx. € 150 million per year.
The Dutch Federation of University Medical Centers (NFU) has designated the Department of Dermatology & Allergology at UMC Utrecht as the National Center of Expertise for patients with difficult-to-treat AD. The center, headed by prof. Marjolein de Bruin-Weller, is heavily involved in clinical and translational AD research and patients from all over the Netherlands are referred to UMC Utrecht because of its specific expertise.
Coco Dekkers, MD (1994, Blaricum) defended her PhD thesis on October 15, 2025 at Utrecht University. The title of the thesis was “A deep dive into the clinical and biological effects of dupilumab and tralokinumab in atopic dermatitis – Insights from real-world studies to optimize treatment”. Supervisors were Prof. Femke van Wijk, PhD (Center for Translational Immunology, UMC Utrecht) en Prof. Marjolein de Bruin-Weller, MD PhD (Department of Dermatology and Allergology, UMC Utrecht). Co-supervisors were Daphne Bakker, MD PhD and Marlies de Graaf, MD PhD (both Department of Dermatology and Allergology, UMC Utrecht). In August 2024, Coco Dekkers started her residency in dermatology at UMC Utrecht.
Previous articles on this subject
December 18, 2024:
Optimizing the treatment of atopic dermatitis
November 22, 2023:
Exploring daily practice performance of dupilumab in atopic dermatitis
November 17, 2023:
PhD project unravels associations between eye disease, atopic dermatitis and antibody treatment
September 21, 2023:
Optimizing management of atopic dermatitis: results from real world studies
October 5, 2021:
Improved patient profiling and prediction of response to immune-modulating treatment in atopic dermatitis