A clinical study in patients with inflammatory bowel disease demonstrates that investigating immunoglobulin A (IgA) responses to microbiota can uncover potential disease-modifying microbial organisms and reveal improved biomarkers of clinical course in IBD. This was concluded in a study by Dutch and American investigators that was published recently in Cell Host & Microbe.
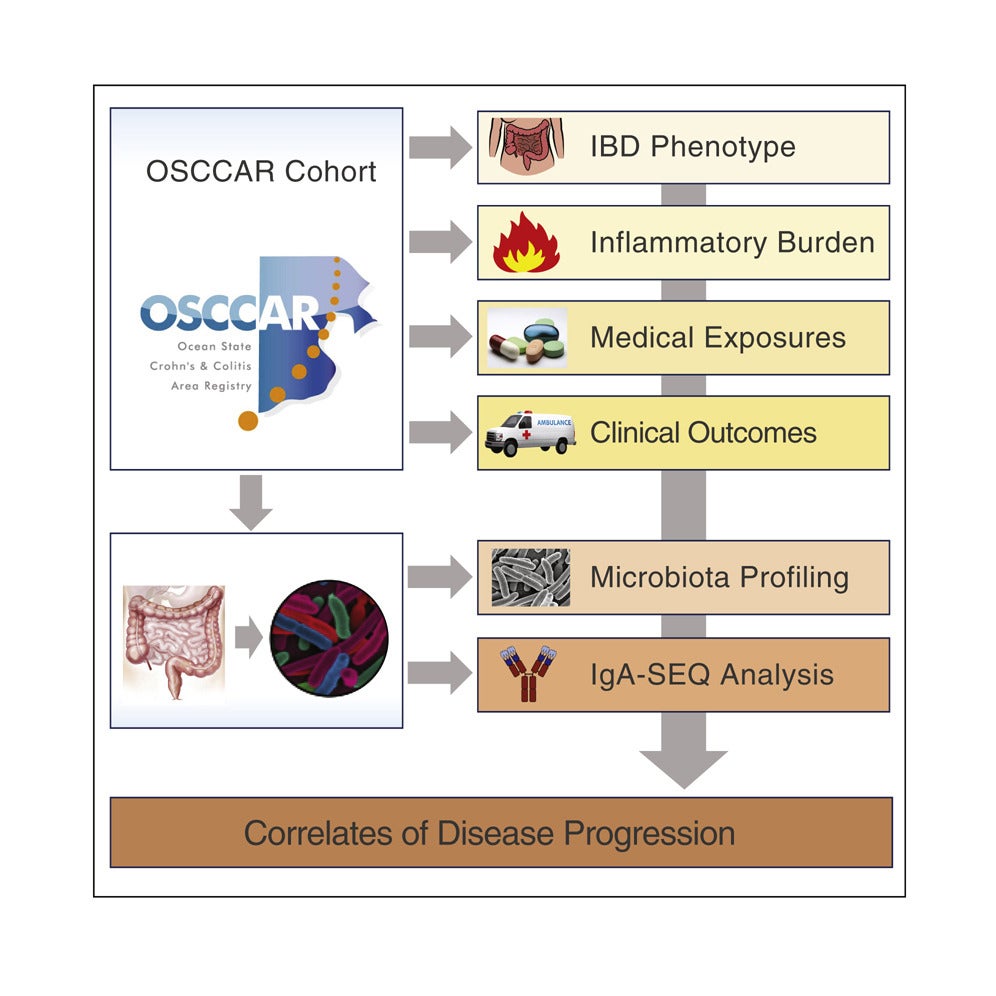
The immunopathogenesis of inflammatory bowel disease (IBD) has been attributed to a combination of host genetics, defects in the innate and adaptive immune system and an imbalance of the intestinal microbiota. This imbalance can take various forms, including overall decreases in microbial diversity, increases of deleterious bacteria, or decreases of protective bacteria. Previous work in a small cohort of IBD patients suggested that pro-inflammatory bacteria are highly coated with secretory immunoglobulin A (IgA).
Using bacterial fluorescence-activated cell sorting, coupled with 16S rRNA gene sequencing, the investigators profiled IgA coating of intestinal microbiota in fecal samples taken within 3 months of IBD diagnosis of 184 IBD patients (115 with Crohn’s disease and 69 with ulcerative colitis) and 32 healthy controls and identified bacteria associated with disease and treatment. Forty-three types of bacteria displayed significantly higher IgA coating in IBD patients compared with controls, including 8 types exhibiting differential IgA coating but similar relative abundance.
The investigators also found that patients treated with anti-TNF-α therapy for management of their IBD exhibited dramatically altered microbiota-specific IgA responses compared with controls. The data suggest that these medications may improve the course of the disease and reduce IgA coating by addressing a core molecular mediator of commensal-driven inflammation in IBD.
The investigators hypothesized that the pattern of IgA coating of the microbiota might correlate with disease progression. Indeed, increased IgA coating of Oscillospira bacteria was associated with a delay in time to surgical intervention. Such findings may serve as a basis for microbiome-based translational biomarkers for the prediction of disease progression.
Co-first author of the paper Marcel de Zoete PhD (Department of Medical Microbiology, UMC Utrecht) concludes: “Our results demonstrate that investigating IgA responses to microbiota in a clinically well-defined cohort of IBD patients can uncover potential disease-modifying microbial organisms and reveal improved biomarkers of the clinical course in IBD.”
IBD describes disorders that involve chronic inflammation of the digestive tract. It includes (a) ulcerative colitis (inflammation and ulcers along the superficial lining of the colon and rectum), and (b) Crohn’s disease (an inflammation of the lining of the digestive tract). Both ulcerative colitis and Crohn’s disease are characterized by diarrhea, rectal bleeding, abdominal pain, fatigue and weight loss. It can be debilitating and sometimes leads to life-threatening complications. It is estimated that the prevalence of IBD in the Netherlands is 1 percent. Moreover, each year about 10,000 people are diagnosed with IBD.
Shapiro JM, de Zoete MR, Palm NW, Laenen NW, Bright R, Mallette M, et al. Immunoglobulin A targets a unique subset of the microbiota in inflammatory bowel disease. Cell Host Microbe 2021;29:83-93.