International research – coordinated by clinical microbiologist and clinician-scientist András Spaan (UMC Utrecht and The Rockefeller University) – has unraveled why, after infection with the notorious bacterium Staphylococcus aureus, some people fall severely ill. In this seminal study, now published in the journal Science, the investigators describe how a genetic condition – called OTULIN haploinsufficiency – predisposes people to life-threatening staphylococcal disease. Using sophisticated methods, the investigators unravel the disease on a molecular level and identify therapeutic leads.
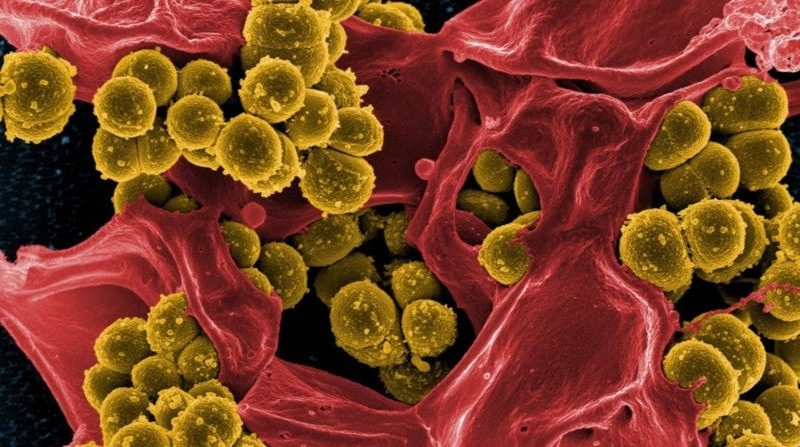
Staphylococcus aureus is a bacterial pathogen impacting human health. There is no vaccine to prevent S. aureus infections. Resistance to commonly used antibiotics, as seen in methicillin-resistant S. aureus (MRSA) strains, makes treatment of these infections increasingly difficult. Most people carry S. aureus asymptomatically on their skin and in their nostrils, but in a small minority the bacterium causes life-threatening disease. This interindividual variability upon infection with S. aureus is not well understood. Investigators from University Medical Center (UMC) Utrecht (the Netherlands) and The Rockefeller University in New York (NY, U.S.A.) set up a study to unravel why, after infection with S. aureus, some people fall severely ill. The scientists used human genetics approaches to study a cohort of patients with unexplained, severe staphylococcal disease. The results of this study have now been published in Science.
First and corresponding author András Spaan (a clinical microbiologist and clinician-scientist at the Department of Medical Microbiology, UMC Utrecht and at the Laboratory of Human Genetics of Infectious Diseases, The Rockefeller University) summarizes: “In our patients with severe staphylococcal disease, we found an enrichment for rare heterozygous mutations in a gene named OTULIN. This gene is located on the short arm of chromosome 5 and encodes for an enzyme. Patients carrying this genetic defect had an increased risk to suffer from episodes of life-threatening necrosis of the skin or lungs. Their disease was typically triggered by S. aureus infections and occurred from adolescence onward.”
It appeared that the disorder operates by haploinsufficiency. Haploinsufficiency is the genetic term to describe a situation that occurs when one copy of a gene is inactivated or deleted and the remaining copy of the gene is not sufficient to produce adequate levels of the gene product. Spaan: “We soon realized that our discovery had implications for patients with a chromosomal deletion syndrome called 5p- (or Cri-du-Chat) syndrome. The deletion of the short arm of one of their chromosomes 5 includes OTULIN in most of the cases, making them haploinsufficient for OTULIN by definition. Indeed, we demonstrated that also these people are predisposed to severe infections due of OTULIN haploinsufficiency.”
Surprisingly, the severe staphylococcal infections in the patients could not be explained by a defect of their white blood cells. The investigators found that OTULIN haploinsufficiency causes an increased susceptibility of tissue-resident, non-white blood cells to the staphylococcal virulence factor α-toxin. Spaan: “By studying the disorder at the genetic, cellular, immunological, and clinical levels, we established causality between this inborn defect and the infectious disease. We discovered that OTULIN governs a key mechanism of cell-intrinsic anti-staphylococcal immunity in humans. Our description of an inborn error of cell-intrinsic immunity to a bacterial pathogen is a first of its kind”.
The team of investigators used human genetics as a compass to clarify the enigma of interindividual variability in the course of staphylococcal infections. While providing seminal scientific insights into the mechanisms of staphylococcal disease, this approach can also pave the way for the development of innovative and personalized anti-infectives. Spaan: “We found that naturally elicited antibodies, targeted against the staphylococcal α-toxin, can protect the patients’ cells and compensate for their inborn error of cell-intrinsic immunity. Our study reveals the potential of developing therapies that interfere with bacterial toxins to prevent or treat infections like those caused by S. aureus.”
Spaan AN, Neehus A-L, Laplantine E, Staels F, Ogishi M, Seeleuther Y, et al. Human OTULIN haploinsufficiency impairs cell-intrinsic immunity to staphylococcal α-toxin. Science, May 19, 2022