The research groups of Jeroen Bakkers and Toon van Veen bring forward the first patient-specific model for the heart disease arrhythmogenic cardiomyopathy (ACM). They were able to recapitulate the human form of this disease by introducing a genetic mutation called Phospholamban (PLN) p.Arg14del in zebrafish. The cellular changes resulting from the mutation could be recovered using the drug istaroxime. Eventually, the findings – published on 9 December in Nature Communications – may contribute to the understanding of the molecular mechanisms of the PLN mutation and the ways in which the symptoms of ACM can be relieved in patients.
Arrhythmogenic cardiomyopathy (ACM) is a type of heart disease that causes a layer of fat to accumulate in the outer layer of the heart, which replaces the healthy heart muscle. This causes changes in the heart rhythm and eventual heart failure due to the inability of the heart to effectively contract. A specific genetic mutation called Phospholamban (PLN) p.Arg14del is known to cause ACM in humans. Given that more than 1.000 people carrying this mutation have been identified, it is very prevalent in the Netherlands. However, the process leading up to the disease is not understood and treatment is currently lacking.
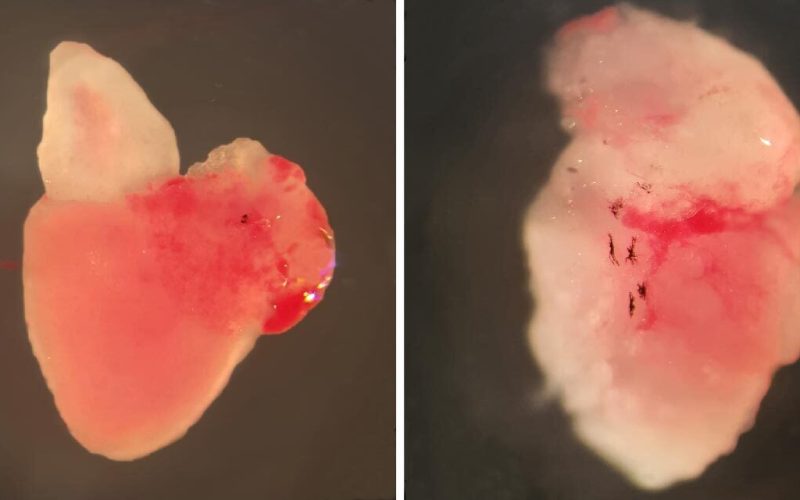
Researchers from the groups of Jeroen Bakkers (Hubrecht Institute/UMC Utrecht) and Toon van Veen (UMC Utrecht) were able to recapitulate the human form of ACM in zebrafish. They induced the PLN mutation in the fish, which caused them to show the same structural changes in the heart as observed in patients with ACM, including the accumulation of fat in the heart. Before these structural changes occured, the researchers observed that the regulation of the calcium levels and action potential duration (APD)was affected in the heart cells of the fish.
“Once we knew we could model ACM by introducing the PLN mutation in zebrafish, we needed a way to rescue the consequent cellular changes thaxst were occurring in the heart,” Sarah Kamel, co-first author of the publication in Nature Communications, explains. She and her colleagues in the group of Toon van Veen treated the fish with istaroxime – a drug that is currently being investigated as a therapeutic for acute heart failure – and discovered that it can successfully recover the calcium levels and APD in affected heart cells. “By using this drug, we were able to recover certain cellular changes in the heart caused by the PLN mutation,” Kamel says.
As previous animal studies were not able to recapitulate the accumulation of fat in the heart in, this zebrafish model provides the first patient-specific model of ACM. This model can be further utilized to study potential disease mechanisms of the PLN p.Arg14del mutation. “If we better understand the disease mechanism, we can further identify key players in the process and hopefully find therapeutics that tackle certain aspects of the mechanism. Eventually, this will hopefully contribute to relieving or even eliminating the symptoms of ACM in patients,” says Lotte Koopman, other co-first author of the publication.
The researchers are continuing the study by looking into the mechanisms that are important for the development of the disease. “We are interested to see whether there are genes that are differentially expressed between fish with and without the PLN mutation. That way, we may be able to identify genes that play a role in the origin of ACM and determine potential disease mechanisms,” Kamel adds. A new research project funded by ZonMW has just started to look into this.
Kamel S.M.*, van Opbergen C.J.M.*, Koopman C.D.*, Verkerk A.O., Boukens B.J.D., de Jonge B., Onderwater Y. L., van Alebeek E., Chocron S., Polidoro Pontalti C., Weuring W.J., Vos M.A., de Boer T.P., van Veen T.A.B., Bakkers J. Istaroxime treatment ameliorates calcium dysregulation in a zebrafish model for phospholamban R14del cardiomyopathy. Nature Communications, 2021.
*These authors contributed equally.
Image showing a healthy adult zebrafish heart (left) and PLN p.Arg14del adult zebrafish heart with arrhythmogenic cardiomyopathy (right). Credit: Sarah Kamel, copyright: Hubrecht Institute.