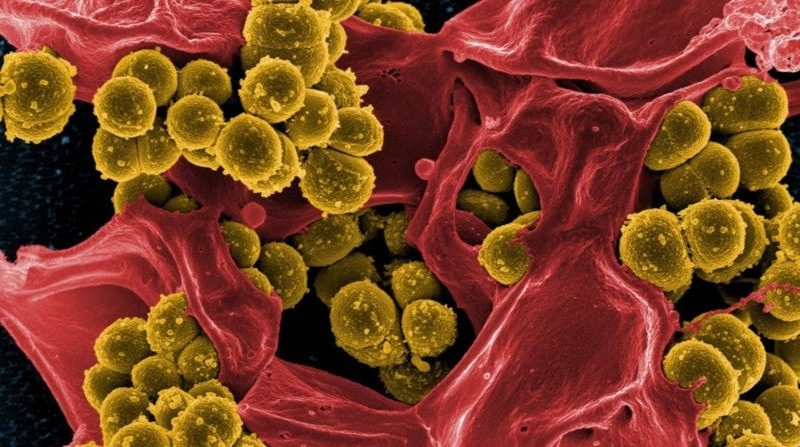
There is considerable evidence that specific polysaccharides (so-called wall teichoic acids or WTA) on the bacterial wall of Staphylococcus aureus might be a good target for vaccination and antibody therapies. This was the overall conclusion that was drawn in the PhD research by Astrid Hendriks (UMC Utrecht), who defended her PhD thesis on January 12 in Utrecht.
Staphylococcus aureus is a leading cause of bacterial infections, including superficial skin infections and bacteremia, which occur both inside and outside the hospital. The emergence of antibiotic-resistant S. aureus strains has increased the need for alternative treatment options, which may be found in boosting host immunity through vaccination. Despite numerous efforts, to this day no effective vaccine exists for S. aureus. It remains incompletely understood what constitutes protective immunity to this bacterium, and which bacterial antigens would be the ideal targets for vaccination. Successful vaccines against other bacterial pathogens, such as the pneumococcal and meningococcal vaccines, target surface-exposed sugars namely capsular polysaccharides that surround the bacterial cell surface. For S. aureus however, this mechanism failed to provide protection through vaccination. Still, other surface-exposed sugars present in the cell wall of S. aureus are potential targets for vaccination or other immune-based therapies. In her thesis, Astrid Hendriks (Department of Medical Microbiology, UMC Utrecht) describes novel insights into the human immune responses to S. aureus wall teichoic acids (WTAs), which are abundantly-expressed cell wall sugars.
S. aureus WTAs exhibit limited heterogeneity, with three different types currently known (WTA glycotypes). Hendriks and her colleagues studied how skin-resident immune cells respond to S. aureus expressing different WTA glycotypes, which is important for rapid activation of the immune system upon S. aureus skin infection. Furthermore, they measured antibody responses to specific WTA glycotypes in healthy individuals and patients with invasive S. aureus infection. It was found that the immune system can recognize S. aureus WTA sugars in several ways, including through antibodies in blood and Langerhans cells in the skin. On the other hand, the bacterium appears to be able to counter recognition by the immune system due to the variation in the composition of the WTA sugars, namely the three different WTA glycotypes. Based on the results obtained from patients with an invasive S. aureus infection, decreased antibody responses to the specific WTA glycotype of an infecting S. aureus strain may contribute to a negative disease outcome.
Astrid Hendriks provides a rationale that boosting immunity to WTA sugars may contribute to protective immunity against S. aureus infections. She concludes that there is considerable evidence that the WTA sugars could be a good target for vaccination and antibody therapies. In addition, she emphasizes not to forget the contribution of local defenses in the skin, since this is where most infections by S. aureus occur.
The cell wall glycopolymer wall teichoic acid (WTA) contributes to bacterial survival, nasal colonization and immune interaction of Gram-positive bacteria such as S. aureus. Human immune receptors from multiple classes recognize glycan modifications on S. aureus WTA. These include surface-expressed receptors on immune cells, scavenger receptors and soluble receptors in serum. Dissection of the functional consequences of WTA–receptor interactions should provide insight into protective versus evasive responses. S. aureus WTA is an interesting target for therapeutic and preventative approaches, such as antibody therapy, phage therapy, and vaccination.
Astrid Hendriks (1992, Utrecht) defended her PhD thesis on January 12, 2022 at Utrecht University. The title of her thesis is “Bittersweet memories – Immunity against Staphylococcus aureus wall teichoic acid”. Supervisors were prof. dr. Jos van Strijp (Department of Medical Microbiology, UMC Utrecht) and prof. dr. Nina van Sorge (Department of Medical Microbiology and Infection Prevention, Amsterdam UMC). Co-supervisor was dr. Fabio Bagnoli (GlaxoSmithKline Vaccines, Siena, Italy). This PhD project was part of DISSection and was funded as a H2020 Marie Curie ITN project. Astrid will continue her career as a post-doctoral researcher at Amsterdam UMC.