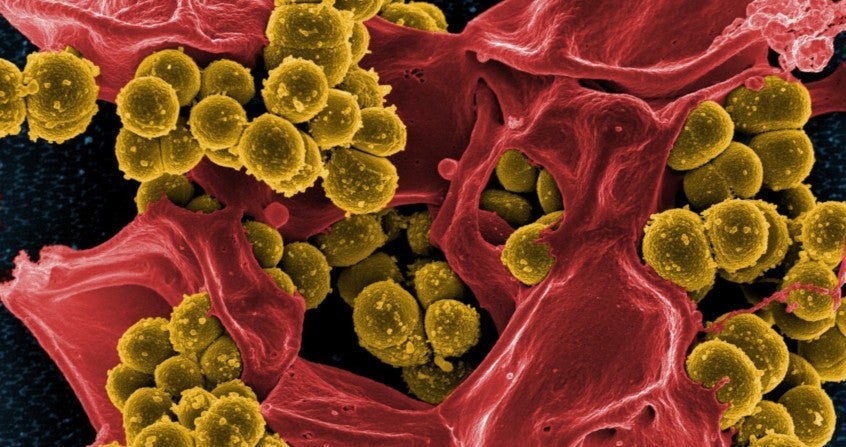
The opportunistic bacterium Staphylococcus aureus can easily develop resistance against antibiotics due to the formation of biofilm, where bacteria are surrounded by a self-made extracellular layer that prevents the antibiotic from reaching the bacterium. PhD research by Lisanne de Vor has shown that antibodies (coupled to specific enzymes) have the potential to degrade biofilm. If confirmed in clinical studies, this could result in development of antibody therapy that would make a resistant bacterium sensitive again for antibiotics.
Staphylococci (such as S. aureus and S. epidermidis) are bacteria that live on the skin and can cause a wide range of infections, such as dermatitis, endocarditis and implant infections. Because of the rise of antibiotic resistance, treatment of these infections is getting increasingly complicated. With respect to staphylococcal infections, biofilm formation on the surface of a medical implant (such as heart valves or prosthetic joints) is another complication. In a biofilm, bacteria are protected from antibiotics and immune cells by a self-made layer of sugars, proteins, and DNA. Because current therapies are often not sufficiently effective against staphylococcal infections, there is a significant medical need to develop alternative treatment strategies, such as antibody therapy.
Biofilm
Antibody therapies are primarily being used for the treatment of cancer or auto-immune diseases, but as of now little is know about their potential in infectious diseases. In her thesis, Lisanne de Vor (Department of Medical Microbiology at UMC Utrecht) explored how antibodies can be used to battle infections with S. aureus and S. epidermidis.
Antibodies can specifically bind to foreign structures, such as bacteria, and label them as ‘dangerous’ for the immune system. In addition, there are no known antibodies that can bind to biofilm. Lisanne de Vor and colleagues have shown that antibodies can recognize staphylococci in biofilm, despite the layer of protection surrounding the bacteria. This means that antibodies could possibly be used for the development of antimicrobial therapies for implant-related infections.
It is known from previous studies that bacteria may become sensitive again to antibiotics and immune cells after being released from a biofilm. Specific enzymes that cleave the protective mucus layer are therefore of great interest, but are difficult to administer to patients. To investigate the potential of antibodies in this respect, the investigators coupled antibodies to these specific enzymes. In an in vitro study, Lisanne de Vor and co-workers found that the antibody-enzyme fusion proteins retained the ability to bind bacteria. The protein was also able to prevent S. aureus biofilm formation and to degrade preformed biofilm.
In another study from her thesis Lisanne de Vor also showed that a modification of antibodies, that was initially developed to boost the immune system against tumor cells, can also be applied to treat staphylococcal infections in vitro. Antibodies with this modification can even activate the underdeveloped immune system of neonates to eliminate bacteria. These findings provide insight that antibodies could enhance phagocytosis by neutrophils and therefore might be useful in the management of neonatal bloodstream infections (sepsis) due to S. epidermidis infection.
Lisanne de Vor concludes: “Our research allows for a better understanding of how antibodies can stimulate the phagocytosis of staphylococci. We have also gained more insights into the use of monoclonal antibodies against biofilm infections. These findings can contribute to the development of antibody therapy against staphylococcal infections.”
Lisanne de Vor (1993, Nieuwegein) defended her PhD thesis on November 4, 2022 at Utrecht University. The title of her thesis is “Staphylococcal infections – can antibodies help?.” Supervisors were prof. dr. Suzan Rooijakkers (Department of Medical Microbiology, UMC Utrecht) and prof. dr. Harrie Weinans (Department of Orthopedics, UMC Utrecht). Co-supervisor was dr. Kok van Kessel (Department of Medical Microbiology, UMCUtrecht). Lisanne will continue her career as a postdoc at the Bacteria & Complement research group of prof. Suzan Rooijakkers at UMC Utrecht.