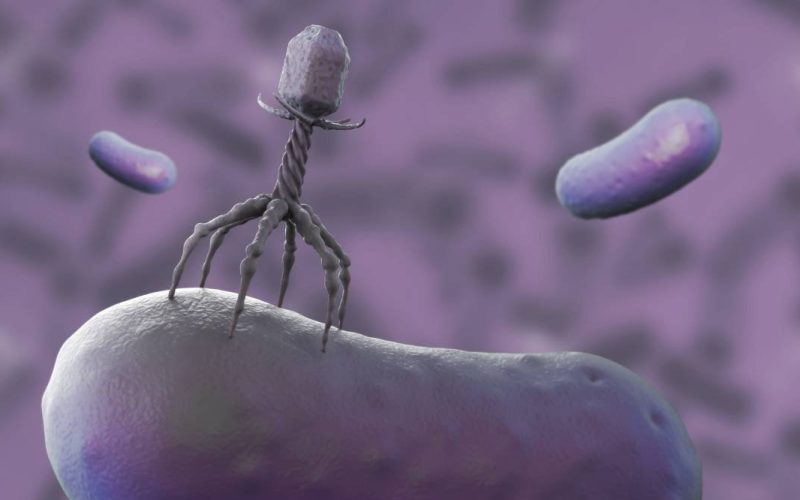
Collaborating researchers from University Medical Center Utrecht and Delft University of Technology have received funding from the Dutch Research Council (NWO) to develop more broadly acting bacteriophages. With help of such adapted phages, the researchers want to overcome bacterial defense barriers, which are one an important mechanisms in the bacterial resistance against bacteriophages.
Bacteriophages (‘phages’) are viruses that can kill bacteria and are considered a promising alternative for the treatment of antibiotic resistant bacterial infections. They have a high specificity for their host bacteria often only being capable of infecting an individual strain of one specific bacterial species. This allows for targeted antibacterial therapy, omitting collateral effects on commensal bacteria. Recently, researchers at TU delft have discovered that clinical strains accumulate defense mechanisms to avoid being killed by phages. For example, clinical strains of the bacterium Pseudomonas aeruginosa carry a diversity of over 100 different defense mechanisms and a single strain may accumulate up to 20 of these in its genome. Fortunately, phages developed a variety of counter-defense mechanisms. In this project, researchers want to combine these counter-defense mechanisms and create a phage that is able to infect a broader range of bacterial strains with different defense mechanisms. This broadening of the activity of bacteriophages is seen as an important step in the clinical application of phage therapy.
The combined research team consists of medical microbiologist Pieter-Jan Haas MD PhD (Department of Medical Microbiology, UMC Utrecht) and microbiologist prof. Stan Brouns PhD (Department of Bionanoscience, TU Delft). Pieter-Jan Haas is project leader and responsible for coordination and communication between the academic researchers. Two postdocs (one in Utrecht and one in Delft) will be assigned to the project for a period of 2 years. In addition, a research technician (partially funded by this grant and trained in phage techniques) is available at UMC Utrecht to provide technical support in phage production, high throughput assays, sequencing and bacterial modifications. For this project, an amount of € 511.754 has been granted.
The knowledge and tools on phage defense systems and phage biology at TU Delft added to the available state-of-the-art readout systems, susceptibility testing and microbiological genome editing tools in a clinical setting at UMC Utrecht creates an unique synergistic combination. The combined skills and knowledge, the availability of clinically relevant P. aeruginosa strains at UMC Utrecht and phages at TU Delft and the translation towards clinical application makes these partners the prime candidates for such an endeavor.
According to the WHO, antimicrobial resistance (AMR) is a global health and development threat and requires urgent multisectoral action. The European Centers for Disease Control (ECDC) estimate that up to 35.000 people in Europe die each year from antibiotic-resistant infections, a number that is expected to increase in the next decades. In addition to death and disability, prolonged illness results in longer hospital stays, the need for more expensive medicines and increased health care costs. Without effective antimicrobials or alternatives, the success of modern medicine in treating infections, including during major surgery and chemotherapy, would be at increased risk.
Study coordinator Pieter-Jan Haas explains: “Tackling ongoing and emerging infectious diseases and AMR requires alternatives to the use of antibiotics, and phage therapy has potential in this area. The increase in antimicrobial resistance and the limited amount of new antimicrobial compounds, renewed the interest in antibacterial use of phages. Before bacteriophages can be broadly applied we need more high quality clinical trials that show the efficacy of phages in anti-infective treatment. The first well-controlled studies using phages to treat infections are underway. However, a deeper understanding of phage-bacteria interaction and how bacteria evade phage infection is essential to select the best phage candidate for these clinical trials. We look forward to start this project and develop a set of adapted bacteriophages that confer broad range activity against clinical P. aeruginosa strains. When successful, the efficacy and safety of these phages may be investigated in clinical trials, for example in patients with P. aeruginosa associated implant infections.”
The Novel Antibacterial Compounds and Therapies Antagonising Resistance (NACTAR) program from NWO – initiated in 2017 – focuses on research into new sources and alternatives to antibiotics. For the 2023 call, a total of nearly 1.6 million euros has been made available together with the Ministry of Health, Welfare and Sport. With this budget, four studies have been selected in which the effect of new antimicrobial agents and methods are validated. This can be an important contribution to curing diseases caused by bacteria against which current drugs and antibiotics no longer work.