In patients with metastatic skin cancer (melanoma), the presence of AI-recognized immune cells in the tumor prior to the start of immunotherapy provides a good prediction of the likelihood of successful treatment. The study also shows that the AI algorithm provides better results than manual counting by pathologists. This is evident from a large-scale, multicenter study by Mark Schuiveling and colleagues at UMC Utrecht.
In this study – now published in JAMA Oncology – researchers analyzed baseline tumor material from 1,202 adult patients with metastatic melanoma (a serious form of skin cancer) who had been treated with immunotherapy between 2016 and 2023. They used an advanced AI algorithm that recognizes tumor-infiltrating lymphocytes (TILs) (a specific type of immune cell that attacks cancer cells) on standard pathological images of tumors.
The study confirmed that the amount of TILs in these images strongly correlates with the likelihood of favorable treatment outcomes during the follow-up period of the study. Patients with more TILs had a 40 percent higher chance of response, a 15 percent lower chance of disease progression, and a 17 percent lower chance of mortality. “The more AI-recognized TILs present in the metastases before the start of treatment, the greater the chance of a good response to immunotherapy, the lower the chance that metastases will start growing again, and the lower the chance of mortality during the follow-up period of the study,” said oncologist in training and PhD student Mark Schuiveling, MD from the Department of Medical Oncology at UMC Utrecht.
Mark Schuiveling, MD PhD
What makes these results important is that the predictive value of TILs appears to be independent of known clinical predictors. In other words, it is independent of the stage the disease is at or what other characteristics the patient has. “This makes AI-based TIL analysis a promising addition to existing tools for making treatment choices in these patients,” says Schuiveling. The study also shows that the AI algorithm provides better results than manual counting of TILs by pathologists. This is because manual assessment is subject to variation between assessors, while AI consistently applies the same method. Because the algorithm works on the standard images that the pathologist uses for making a melanoma diagnosis, this technique is widely applicable in the clinic without additional steps or expensive additional staining.
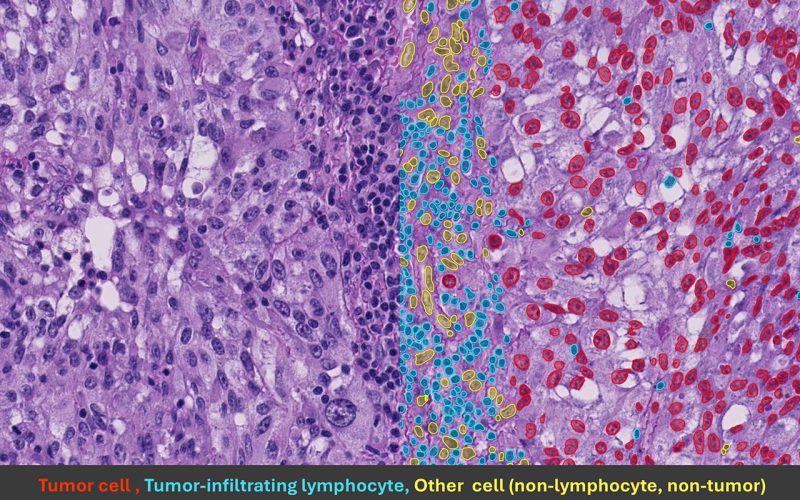
The picture above shows a melanoma biopsy with results from the AI model. In red are tumor cells, in blue TILs and in yellow non-tumor and non-TILs.
Nevertheless, according to the researchers, there is still room for improvement. The model is already good at identifying patients who are expected to respond well to immunotherapy, but it cannot yet predict with 100 percent certainty who won’t respond at all. In addition, the technology could potentially be used to reduce treatments in patients with a favorable profile, thereby limiting side effects and health care spending. This is part of the follow-up research that Schuiveling is currently working on. This focusses on AI techniques that, for example, measure the distance between these immune cells and tumor cells, or models that can analyze the entire microscopic image at once.
The research – the largest trial to date using AI to quantify TILs in advanced melanoma – is part of the PREMIUM project that is coordinated by UMC Utrecht and TU Eindhoven in collaboration with 10 other Dutch melanoma referral centers. The images used came from pathology archives with a variety of staining techniques, which increases the applicability of the AI model in practice. Furthermore, the researchers have made the AI model used, together with the development data, freely available so that others can also use the model. In this way, this research also contributes to transparency and reproducibility in science. “We trust that our results will contribute to a future in which we can better predict which patients will truly benefit from immunotherapy,” says Mark Schuiveling. “With the smart use of AI, we can better tailor treatment to the individual, prevent unnecessary side effects, and save money by spending less on expensive drugs.”
This study is part of the Cancer and Infection & Immunity strategic research programs at UMC Utrecht, in which researchers and healthcare professionals collaborate to improve the diagnosis and treatment of diseases in which the immune system plays a role. The research is made possible due to financial support of the Hanarth Fund, Philips and ZonMw and logistical support of Palga, the Dutch Nationwide Pathology Databank.
Schuiveling M, van Duin IAJ, ter Maat LS, van der Weerd JC, Verheijden RJ, van den Berkmortel F, Blank CU, Breimer GE, Burgers FH, Boers-Sonderen M, van den Eertwegh AJM, de Groot JWB, Haanen JBAG, Hospers GAP, Kapiteijn E, Piersma D, Vreugdenhil G, Westgeest H, Schrader AMR, Pluim JPW, van Diest PJ, Veta M, Suijkerbuijk KPM, Blokx WAM. AI-detected tumor-infiltrating lymphocytes and outcomes in anti-PD1 based treated melanoma. JAMA Oncology, published online October 16, 2025
July 15, 2025
Optimizing efficacy and toxicity of cancer immunotherapy – a delicate balance
July 24, 2024
The wonderful world of immunotherapy
March 21, 2024
Focus on side effects of immunotherapy