UMC Utrecht has received a grant of 4 million euros for the first clinical study in the Netherlands involving a customized therapy with bacteriophages for patients with recurrent urinary tract infections. This represents a unique collaboration between the departments of microbiology, infectious diseases, pharmacy, and urology at UMC Utrecht. The grant has been awarded from the Promising Care subsidy scheme of the National Healthcare Institute and ZonMw.
Patients with a recurrent urinary tract infection (RUTI) can participate in the study, although it will be at least a year before it is ready to include patients. The main cause of urinary tract infections (such as cystitis) is the intestinal bacterium E. coli. Approximately one in five women suffers from such an infection at least once a year, causing many complaints and sick leave. Professor of Urology Laetitia de Kort, MD PhD explains: “We usually treat RUTIs with antibiotics, but repeated use of these drugs means that they are increasingly ineffective due to antimicrobial resistance (AMR). We therefore urgently need an alternative for these patients. A therapy using bacteriophages, for example, could therefore be an interesting option.”
Internist-infectiologist Marjolein Hensgens, MD PhD says that an estimated 1,250 patients in the Netherlands with RUTIs who do not respond well to treatment due to AMR are eligible for alternative therapy each year. “For these patients, there are no longer any antibiotics available that they can take in pill or liquid form. They then have to be given antibiotics intravenously during a hospital stay. If phage therapy would work, we can treat these patients better, significantly reduce antibiotic use, and save on healthcare costs.”
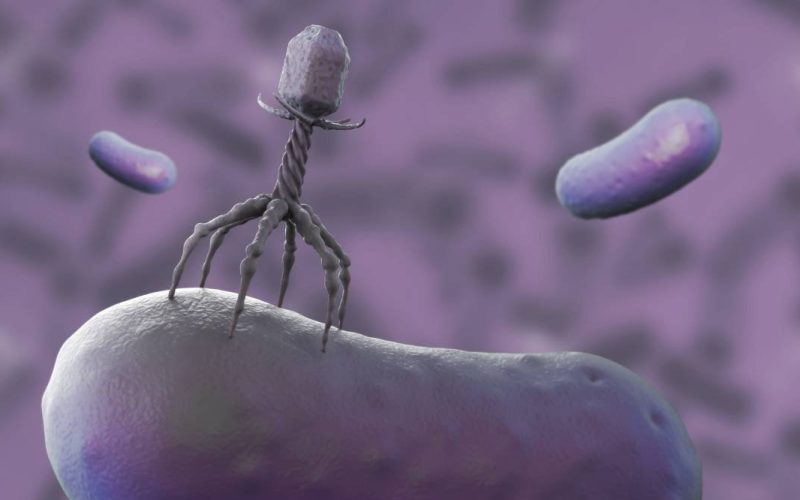
Bacteriophages are viruses that can kill bacteria. There are countless different types. A bacteriophage works very specifically on one type of bacterium. The advantage of bacteriophages is that they could also work against bacteria that have become resistant to antibiotics. This is becoming increasingly common, particularly in bacteria that cause urinary tract infections, as a recent report by the National Institute for Public Health and the Environment (RIVM) has shown. People who carry such resistant bacteria usually cannot get rid of them. These bacteria cause increasingly frequent and severe infections, posing a growing threat to public health.
“What is special about this study is that, for the first time, we will select the exact phage that is appropriate for the bacteria carried by the patient,” says medical microbiologist Pieter-Jan Haas, MD PhD. The study will be conducted on 150 people. Half will receive personalized phage therapy, while the other half (the control group) will receive only a saline solution. After four weeks, we will check for the first time whether there is any difference.
Bacteriophage therapy is already being used in other parts of the world, but mostly as a cocktail of multiple bacteriophages. “Then it’s a matter of waiting to see if the right ones are among them,” says Haas. “That’s why we’re still not really sure how well bacteriophages work against resistant bacteria. With this study, we are exploiting the full potential of bacteriophages, and it is the first time that scientific research is being conducted on this scale.”
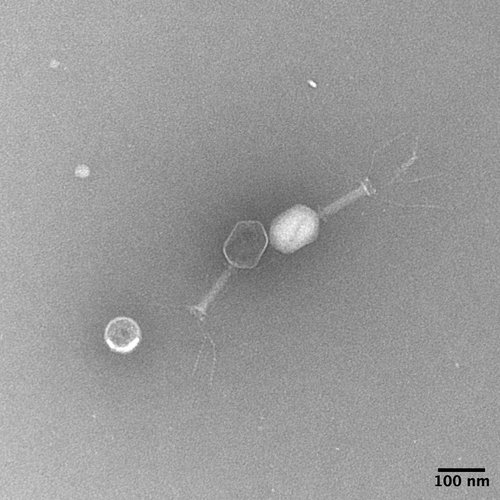
Electron microscopy image of a Klebsiella targeting bacteriophage (courtesy of KlebPhaCol.org)
In order to obtain the right bacteriophages, it is essential to be able to produce bacteriophages at UMC Utrecht itself. That is why a significant portion of the grant will go toward setting up the production of bacteriophages for medicinal use according to good manufacturing practice (GMP). Pharmacist Jan Dekker, MSc MBA is leading that part of the project. “In fact, it is the production of a medicine: you want to do that properly and safely. And always according to the same procedures.” Although bacteriophages have been considered a promising medicine for at least a century, the production process is still in its infancy. A procedure established at European level that guarantees the required quality of phage therapy production (according to GMP standards) has only been in place for 1.5 years.
Due to the complex and unique nature of the PhageR-UTI study, specific knowledge and expertise is required from the departments of microbiology, infectious diseases, pharmacy, and urology at UMC Utrecht. In addition, the project is supported by patient experience experts, methodologists and statisticians, a health economist, and an implementation scientist.
Core team PhageR-UTI project (left-to-right): Renske ten Ham, Kim Romijnders, Jan Dekker, Marjolein Hensgens, Pieter-Jan Haas, Laetitia de Kort, Henri van Werkhoven and Annemarie Verschoor
Previous articles on this subject: