We are fascinated by the molecular interplay between bacteria and the human immune system. We aim to provide a scientific understanding of the mechanisms by which antibodies and complement induce bacterial immune clearance and contribute to development of anti-bacterial immunotherapies. Below you can find our three main research themes.
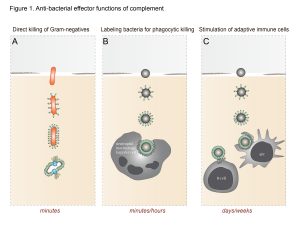
Human immune protection against invading bacteria critically depends on the bactericidal actions of the complement system. Complement, a large network of proteins in plasma and body fluids, triggers a variety of responses that help to kill the bacterium. The most rapid response is the formation of ring-structured pores (the Membrane Attack Complex (MAC)) that directly kill Gram-negative bacteria within minutes. Furthermore, complement activation results in labeling of bacteria for phagocytosis and intracellular killing by immune cells.
Our lab has strong expertise at the scientific crossroads of bacterial infections and complement biology. We aim to generate novel tools to study complement activation on bacteria and provide a better scientific understanding of the mechanisms by which complement induces bacterial immune clearance.
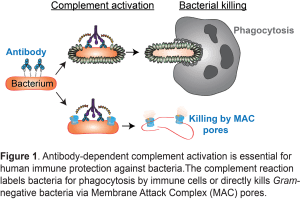
An important mechanism by which antibodies accelerate bacterial killing is by potent activation of the complement cascade. To do so, antibodies should bind bacterial cells via their variable (Fab) domain and subsequently trigger Fc-mediated complement activation.
The ability of complement to kill bacteria could be exploited for effective antibacterial therapies. However, identification and therapeutic development of ‘complement-enhancing’ antibodies is severely hampered by our limited knowledge of the processes underlying antibody-dependent complement activation against bacteria. For instance, we still don’t understand why certain antibodies induce bacterial killing and others don’t.
Our lab aims to provide mechanistic insights into antibody-mediated immune clearance on bacterial cells. In addition, we recently developed novel approaches to discover anti-bacterial antibodies from human B cells.
Although crucial for local clearance of infections, erroneous activation of complement on the body’s own cells is the root cause of many serious inflammatory diseases, including high medical need and orphan indications. While a few complement-blocking drugs have entered the market, pharmaceutical companies continue to search for highly specific complement inhibitors for treatment of a variety of inflammatory diseases while minimizing the risk to infections.
We aim to gain insight into the effect of complement inhibition on certain anti-bacterial defence mechanisms. In addition, our lab has recently developed complement-targeting nanobodies to detect and block complement activation.