HARNESS PROJECT
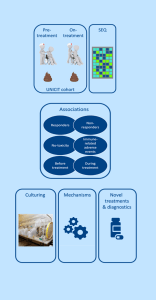
Immune checkpoint inhibitors (ICI) have revolutionized cancer treatment strategies. A number of previously untreatable (metastatic) cancers became treatable by (re)activating the anti-tumor immune response. Surprisingly, the intestinal microbiota plays an important role in the success rate of ICI therapy in cancer patients, showing the crosstalk between the immune system and the bacteria within the intestinal tract.
Unfortunately, ICI therapy is not effective for all cancer patients and can result in (severe) toxicity. Research shows that certain bacteria can enhance ICI therapy response but can also drive intestinal inflammation and thereby toxicity. In this project we aim to uncover intestinal bacteria that impact ICI therapy response and toxicity and subsequently unravel the underlying mechanisms. Ultimately this will provide a rationale for microbiota-targeted therapy to optimize the effectiveness of ICI therapy in cancer patients.

MISTAR PROJECT
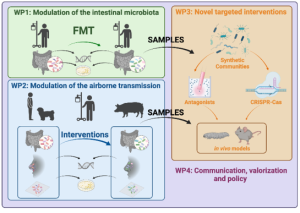
MISTAR is a collaborative project between Utrecht University, the University of Birmingham, the Ramón y Cajal Health Research Institute, the University of Antwerp, the University of São Paulo and the University of Exeter and is funded by JPIAMR and the European commission. This project sets out to monitor and control the selection and spread of Antibiotics Resistance Bacteria (ARB) and Antibiotics Resistance Genes (ARG) in hospitals and farms by developing and implementing new intervention strategies.
The De Zoete lab coordinates the project and aims to expand our gut bacterial biobank through culturomics to identify commensal bacteria that possess relevant inhibitory activity towards ESKAPEs bacteria, a group of virulent and antibiotic resistant pathogens. Furthermore, the mechanism of inhibition of inhibitory bacteria will be investigated.
Visit website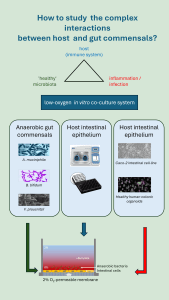
HOST – GUT COMMENSAL INTERACTIONS
In our gut, the host and microbiota are in close contact: only a single layer of epithelial cells separates bacteria in the intestinal lumen from entering the body. A close symbiotic relationship between the gut microbiota and the host exists, in which bacterial composition and function is tightly connected with human physiology and to health status.
In an infectious or inflammatory context, often pathogenic bacteria are studied. At the same time, “health-promoting” commensal bacteria, which are abundantly present in the gut, can positively affect host intestinal epithelial cells and underlying immune cells both directly and indirectly. We are interested in these host – gut commensals interactions, and in the immunomodulatory effects in the context of intestinal inflammation.
To achieve this, we aim to optimize in vitro host-microbiota models that enable the co-culture of anaerobic gut bacteria with different human cells mimicking physiologically relevant conditions, with a focus on oxygen levels and mucus production. With this model, commensal strains will be extensively examined to further unravel the complex interactions between the host and gut commensals.