Real-world insights shaping the future of targeted treatments for chronic skin diseases.
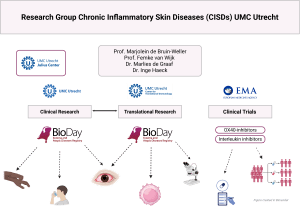
The BioDay Registry is a prospective, multicenter observational registry that systematically collects real-world evidence (RWE) on the use, effectiveness, and safety of targeted systemic treatments in both adult and pediatric patients with atopic dermatitis (AD) and prurigo nodularis (PN). Coordinated by the University Medical Centers of Utrecht (UMCU) and Groningen (UMCG), BioDay brings together 20 dermatology centers across the Netherlands, including both academic and non-academic hospitals with expertise in AD and/or PN. The registry is currently expanding internationally, with the planned inclusion of hospitals in Brazil and Belgium.
 Why BioDay?
Why BioDay?AD and PN are chronic, inflammatory skin diseases that can severely impact the quality of life. While AD is the most common chronic skin disease in the Netherlands, affecting over 400,000 people, PN is a rarer, but highly burdensome disease, characterized by intensely itchy nodules. For both diseases, treatment options have historically been limited, especially in moderate-to-severe or therapy-resistant cases. In recent years, a new generation of targeted systemic therapies such as biologicals (e.g. dupilumab, lebrikizumab, tralokinumab) and Janus kinase inhibitors (JAKi; e.g. abrocitinib, baricitinib, upadacitinib) have become available. These treatments offer promising efficacy results and a favorable safety profile in randomized clinical trials (RCTs).
However, RCTs have limitations: they often exclude older patients, young children, those with comorbidities, or those who failed previous treatments. As a result, their findings may not fully reflect treatment effectiveness and safety in daily clinical practice. BioDay addresses this evidence gap by collecting real-world, longitudinal data across a wide range of patient populations.
Ultimately, by bridging the gap between clinical trials and routine clinical care, BioDay aims to contribute to more personalized, safe, and cost-effective treatment for patients with AD and PN.
The primary goals of BioDay are:
Secondary, BioDay aims to:
Visit www.bioday.nl for information on inclusion rates of AD and PN patients, participating hospitals, and the BioDay team, or contact us via bioday@umcutrecht.nl to learn how to participate, or collaborate.
All patients treated with biologics or JAKi for AD or PN in one of the BioDay centers are eligible for inclusion in the registry. The only exclusion criterium is the inability to understand the patient’s information or provide written informed consent.
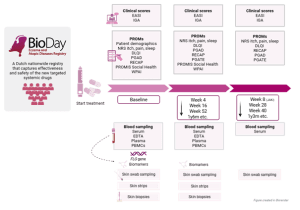
At each scheduled visit (baseline, week 4 and 8 (for JAKi only), and every 3–6 months thereafter), BioDay captures standardized data summarized in the figure below.
BioDay is supported by a consortium of academic and regional hospitals, patient representatives from the Dutch association for people with AD (VMCE) and collaborates with several partners, including the Center for Translational Immunology and the Julius Center of Health Science. The registry operates with long-term infrastructure support and complies with national data protection regulations.
Patient inclusion in BioDay follows routine clinical practice. Treatment decisions are made through shared decision-making between patients and treating physicians, based on national guidelines. BioDay does not alter standard care but supplements it with structured data collection to ensure consistency and comparability.
Importantly, BioDay is a flexible platform: additional modules (e.g., asthma monitoring, conjunctivitis evaluation, hand eczema modules) can be added to the registry as new research questions emerge or new therapies become available.