Patient-based Airway Disease Modeling: from Primary Ciliary Dyskinesia to Colorectal Cancer Lung Metastases
By leveraging advanced culture systems, including primary airway epithelial cells, 3D airway organoids, and high-throughput functional assays, we investigate the mechanisms underlying airway diseases and work toward personalized treatment strategies. Our research focuses on the pathophysiology and therapeutic targeting of both Primary Ciliary Dyskinesia (PCD), a rare genetic disorder, and colorectal cancer lung metastasis, a clinically challenging manifestation of cancer progression. Combining expertise in stem cell biology, regenerative medicine, and translational modeling, our interdisciplinary team develops and applies patient-specific models to drive innovation in airway disease research and therapy development.
Research focus
Our research line currently focuses on three interrelated topics:
PCD is a rare, inherited disorder that impairs the function of motile cilia, tiny hair-like structures that line the respiratory tract and help clear mucus and pathogens from the airways. In PCD, these cilia fail to beat properly, leading to chronic respiratory infections, bronchiectasis, and other lifelong complications starting from birth. Although more than 50 genes have been linked to PCD, no curative treatments exist today. The condition is not only genetically complex but also highly variable in how it presents clinically, which makes developing one-size-fits-all therapies nearly impossible.
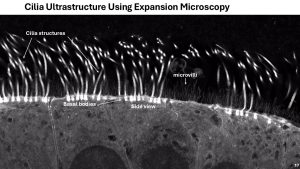
Our group addresses this by developing advanced in vitro models using airway epithelial cells derived directly from PCD patients. These models include differentiated epithelial cultures and 3D airway organoids that mimic the architecture and function of native airway tissue. To understand how ciliary function varies between individuals, we employ a patient-specific, multi-assay in vitro platform that allows detailed assessment of different aspects of ciliary physiology.
In addition to functional analysis, we investigate cilia structure using cutting-edge imaging techniques such as expansion microscopy and lattice light-sheet imaging, providing high-resolution insights into ultrastructural defects caused by specific genetic mutations (in collaboration with Kapitein lab).
These patient-specific models offer a platform to study how genetic mutations translate into structural and functional defects, enabling the identification of genotype–phenotype relationships. They also serve as powerful tools for therapeutic testing, allowing us to evaluate mRNA-based treatments and gene therapy vectors in a preclinical patient-tailored context, bringing us closer to individualized care for people with PCD.
For optimal preclinical-clinical collaboration, we work closely with clinicians Tamara Paff (UMC Utrecht) and Eric Haarman (Amsterdam UMC).
Assistant Professor/Facility manager Utrecht Platform for Organoid Technology
Pediatric Pulmonologist
PhD student
PhD student
Technician
Technician
Colorectal cancer (CRC) is a major contributor to cancer-related mortality worldwide, with death most often resulting from the formation of distant metastases. While the liver is the most common site, lung metastases represent a significant clinical challenge and are associated with poor prognosis. Despite their impact, little is known about how the unique lung microenvironment influences metastatic tumor behavior and response to therapy.
In this project, we focus specifically on lung metastasis of CRC, developing advanced organoid-based co-culture systems that replicate the lung microenvironment. These models are designed to study how the lung-specific niche affects tumor growth, invasion, and treatment response. We hypothesize that the lung microenvironment not only shapes metastatic behavior but also creates specific therapeutic vulnerabilities that can be exploited.
Assistant Professor/Facility manager Utrecht Platform for Organoid Technology
Head Laboratory Translational Oncology/ Chair Utrecht Platform for Organoid Technology
Technician

The Utrecht Platform for Organoid Technology (UPORT) is a central research facility within the UMC Utrecht that enables the development of ‘living biobanks’, collections of long-term, expandable human tissue models derived from healthy and diseased donors. These biobanks are established using cutting-edge technologies such as organoid culture and induced pluripotent stem cells, offering powerful tools for disease modeling, drug testing, and personalized medicine.
UPORT provides a streamlined and ethically approved infrastructure for the standardized acquisition and processing of patient-derived materials, including biopsy samples and surgical tissues. By integrating clinical data with high-quality tissue models, UPORT creates a unique bridge between patient care and research. Our mission is to accelerate translational research with fresh human tissue by:
UPORT serves as a platform for researchers, clinicians, and industry partners who aim to advance precision medicine through next-generation human tissue models.
Visit website and UPORT Team






